
ORIGINAL RESEARCH
published: 19 March 2020
doi: 10.3389/fevo.2020.00056
Frontiers in Ecology and Evolution | www.frontiersin.org 1 March 2020 | Volume 8 | Article 56
Edited by:
Cesar A. Cardenas,
Instituto Antártico Chileno
(INACH), Chile
Reviewed by:
Hugo Mathé-Hubert,
Université de Lorraine, France
Alison Karley,
The James Hutton Institute,
United Kingdom
Christoph Vorburger,
Swiss Federal Institute of Aquatic
Science and Technology, Switzerland
*Correspondence:
Julia Ferrari
†
These authors have contributed
equally to this work
‡
Present address:
Melanie R. Smee,
Microbiology Department, Cornell
University, Ithaca, NY, United States
Specialty section:
This article was submitted to
Population and Evolutionary
Dynamics,
a section of the journal
Frontiers in Ecology and Evolution
Received: 18 October 2019
Accepted: 26 February 2020
Published: 19 March 2020
Citation:
Heyworth ER, Smee MR and Ferrari J
(2020) Aphid Facultative Symbionts
Aid Recovery of Their Obligate
Symbiont and Their Host After Heat
Stress. Front. Ecol. Evol. 8:56.
doi: 10.3389/fevo.2020.00056
Aphid Facultative Symbionts Aid
Recovery of Their Obligate Symbiont
and Their Host After Heat Stress
Eleanor R. Heyworth
†
, Melanie R. Smee
†‡
and Julia Ferrari
*
Department of Biology, University of York, York, United Kingdom
Environmental conditions affect insect fitness, with many species constrained by specific
temperature ranges. Aphids are limited to temperate climates and it is hypothesized
that this is partly due to their heat-susceptible obligate nutritional symbiont Buchnera.
Aphids often carry additional facultative symbionts which can increase the host’s fitness
after heat stress. Here we used the pea aphid (Acyrthosiphon pisum) and three of
its facultativ e endosymbionts (Candidatus Regiella insecticola, Candidatus Fukatsuia
symbiotica (X-type; PAXS), and Candidatus Hamiltonella defensa) to investigate how
these species respond to heat stress and whether their presence affects the fitness of
the host or the obligate symbiont. We exposed aphid lines to a single high temperature
event and measured lifetime fecundity and population densities of both obligate and
facultative symbionts. Heat shock reduced aphid fecundity, but for aphids infected with
two of the facultati ve symbionts (Regiella or Fukatsuia), this reduction was less than in
uninfected aphids. The population density of Buchnera was also reduced after heat
shock, and only recovered in aphids infected with Regiella or Fukatsuia but not in
uninfected aphids or those with Hamiltonella. Although heat shock initially reduced the
densities of two of the facultative symbionts (Hamiltonella and Fukatsuia), all facultative
symbiont densities recovered by adulthood. Two of the facultative symbionts tested
therefore aided the recovery of the obligate symbiont and the host, and we discuss
possible underlying mechanisms. Our work highlights the beneficial effects of protective
symbionts on obligate symbiont recovery after heat stress and how facultative symbionts
may affect the wider ecological community.
Keywords: Acyrthosiphon pisum, Buchnera aphidicola, facultative symbiont, heat stress, insect symbionts,
quantitative PCR, symbiosis
INTRODUCTION
It is well-established that infection with bacterial symbionts can affect an insect host’s biology.
Reproductive fitness, insect behavior, immune pathway function, and responses to natural
enemies may all be influenced by the presence of endosymbionts (
Dion et al., 2011; Gerardo
and Parker, 2014; Vorburger, 2014; Martinez et al. , 2015). By improving the ecological
fitness of a host through raising its immunity to natural enemies, or by enhancing its
tolerance to environmental stress, a vertically transmitted symbiont increases its own fitness
(
Oliver et al., 2005; Brownlie and Johnson, 2009).

Heyworth et al. Symbionts Aid Recovery From Heat-Shock
Rising global temperatures are already affecting insect
populations; there is evidence of range shifts (Parmesan and
Yohe, 2003), changes in phenology (Walther et al., 2002) and
interactions with predators and parasitoids (Harrington et al.,
1999; Schmitz and Barton, 2014
). For several insect groups
infection with various bacterial symbionts has been shown to
enhance resistance to temperature stress (Corbin et al., 2017).
These effects may be direct symbiont-mediated host protection
(Montllor et al., 2002; Neelak anta et al., 2010; Brumin et al., 2011)
or indirect effects of temperature on the symbiont itself (Chen
et al., 2009; Bordenstein and Bordenstein, 2011).
There are a number of hypotheses for the mechanisms
underlying indirect symbiont-mediated protection from heat.
Infection with symbionts has been shown to incre ase the
expression of immune system genes (Laughton et al., 2013),
and it is hypothesized that this immune response controls the
bacteria, restricting growth or location and protecting the host
from microbial over-proliferation (
Kwong et al., 2017; Maire
et al., 2018). It may also bestow temperature tolerance as a by-
product. For example, infection of Rickettsia in whiteflies leads
to the upregulation of stress-response genes in the host and
thus increases survival of the insect under heat shock (Brumin
et al., 2011); similarly, insects infected with bacterial symbionts
often produce more immune cells than t h ose that are uninfected
(Schmitz et al., 2012; Weiss et al., 2012; Laughton et al., 2013; Kim
et al., 2015). There are close links between insect responses to heat
and to infection–many heat shock proteins are chaperones that
aid protein production and refolding post-stress, and they may
also enhance immune responses (Young et al., 1993).
Instead of indirectly affecting the host’s stress or immune
responses, symbionts may themselves produce and release heat
shock proteins or metabolites that directly protect the host or
other microbes that the host depends on (i.e., obligate symbionts)
(Burke G. et al., 2010). Obligate symbionts are often a thermal
“weak link” and more susceptible to temperature extremes than
their hosts (Corbin et al., 2017; Shan et al., 2017; Zhang et al.,
2019). Shielding an obligate symbiont from thermal damage
would therefore benefit both the host and all of its symbionts. For
example, in pea aphids that experience heat shock, the density of
the obligate nutritional symbiont Buchnera aphidicola is usually
reduced, but is maintained at near normal levels in aphids that
carry the facultative symbiont Candidatus Serratia insecticola
(hereafter Serratia) (
Burke G. et al., 2010). It is also plausible
that facultative symbionts might be directly protecting the host
by replacing an obligate symbiont t hat is no longer able to
perform its function. When the obligate symbiont Buchnera is
removed using antibiotics at benign temperat ures, Serratia in pea
aphids moved into the bacteriocytes vacated by Buchnera and
subsequently allow the stressed aphid to survive and reproduce
(Koga et al., 2003, 2007).
Pea aphids, Acyrthosiphon pisum, are a model system for
understanding how facultative symbionts protect their hosts
from thermal stress. They and their obligate symbiont are
typically intolerant to heat in laboratory populations (
Dixon
et al., 1987; Dunbar et al., 2007
), but three of t heir eight potential
facultative symbionts (Serratia, Candidatus Fukatsuia symbiotica
and Candidatus Hamiltonella defensa; hereafter Fukatsuia and
Hamiltonella, respectively) are known to improve survival or
reproduction after heat shock (
Montllor et al., 2002; Koga et al.,
2003; Russell and Moran, 2006; Heyworth and Ferrari, 2015). The
obligate symbiont Buchnera synth esizes essential amino acids
for the host, which are required for aphids to thrive on their
imbalanced diet of plant phloem sap (
Douglas, 1998). Buchnera
has a highly reduced genome (Moran, 1996; Gómez-Valero et al.,
2007) and some genotypes are susceptible to hig h temperatures;
under heat st ress, just five protective heat shock proteins are
deployed (Wilcox et al., 2003) compared to over 75 in its free
living relative Escherichia coli (Carruthers and Mi nion, 2009) and
during severe heat shock Buchnera can be killed.
While the costs and benefits of infection are being explored
in a broad spectrum of insect species, relatively little is known
about how different facultative symbionts confer increased
heat tolerance to their hosts, and how these mechanisms vary
depending on symbiont species. Understanding how insects
can and will respond to increases in temperature is vital to
accurately model current and future populations. We investigate
whether three common facultative symbionts of the pea aphid
[Candidatus Regiella insecticola (hereafter Regiella), Fukatsuia
and Hamiltonella] protect the host and how they respond to heat
stress themselves. Importantly, we test whether t he protection
from the effects of heat co-occur with the protection of the
obligate symbiont Buchnera. We test whether the facultative
symbionts directly protect Buchnera, allow it to recover after heat
stress or protect the host by replacing Buchnera and whether this
mode of protection is similar for all tested facultative symbionts.
MATERIALS AND METHODS
Aphids and Symbionts
Rapid, asexual reproduction results in clonal lines of aphids that
can be kept indefinitely under long-day conditions. This allows
the manipulation of facultative symbiont presence through
antibiotic curing or artificial infections while maintaining
an essentially identical aphid genotype. Two pea aphid
genotypes were used for this study, both collected from the
UK (Supplementary Table S1). Genotype 218 was collected
naturally infected with Fukatsuia and Hamiltonella, and was
cured more than a year before use. This was achieved by
feeding young aphids with broad bean leaves suspended in a
tube of antibiotic solution (0.5% Gentomicin, 1% Ampicillin,
0.5% Cefotaxime in distilled water) over 4 days (
McLean
et al., 2011). Genotype 2 00 was collected naturally uninfected,
harboring no known facultative symbionts. All aphid lines
were screened for Hamiltonella, Regiella, Serratia symbiotica,
Fukatsuia, Spiroplasma sp., Rickettsia sp., and Rickettsiella viridis
following protocols in Tsuchida et al. (2010) and Ferrari et al.
(2012) to ensure that they had the appropriate symbiont
infection and were not infected with any other known facultative
symbionts. The symbiont infections were regularly checked
to detect possible contamination. The symbiont-specific PCR
primers can be found in Supplementary Table S2. The PCR mix
comprised 6.25 µl BioMix (Bioline), 0.1 µl (20 µM) of forward
and 0.1 µl (20 µM) reverse primer, 5.55 µl distilled water and 1.0
µl sample DNA. The PCR reaction was performed at 94
◦
C for
Frontiers in Ecology and Evolution | www.frontiersin.org 2 March 2020 | Volume 8 | Article 56

Heyworth et al. Symbionts Aid Recovery From Heat-Shock
2 min, followed by 35 cycles of: 94
◦
C for 30 s, 55
◦
C for 30 s, and
72
◦
C for 1 min. It concluded with 6 min at 72
◦
C and then cooled
the sample to 4
◦
C indefinitely. PCR products were run on a 1%
agarose gel and t h e presence of a band confirmed the presence of
the symbiont.
Five aphid lines were used in the experiment, two uninfected
with facultative symbionts (200 and 218) and the remainder
infected singly with one of three facultative symbionts, Regiella,
Fukatsuia, and Hamiltonella (Supplementary Table S1). Regiella
was injected into aphid genotype 200, while the other two
symbionts were injected singly into genotype 218. These five
aphid lines comprise three pairwise comparisons between
uninfected and infected aphids, with the uninfected line of 218
used in two comparisons. This design aimed to compare host
fitness and symbiont densities within each pair across the same
aphid genetic background and thus we conducted no analyses
across multiple pai rs. These specific isolates of symbiont were
chosen because preliminary results indicated that they were likely
to provide heat shock protection.
To produce these infections of Hamiltonella, Regiella, and
Fukatsuia we used hemolymph injections from infected donor
aphids (Supplementary Table S1). Hemolymph was extracted
from donor aphids under a microscope using glass needles
and re-injected into the appropriate aphid line and surviving
aphids raised to adulthood. Glass needles were pulled from Kwik-
Fil
TM
borosilicate glass capillaries (1B100-4, World Precision
Instruments, 1 mm diameter) using a P-97 Flaming/Brown
micropipette Puller (Sutter Instrument Co.). The offspring of
the surviving aphids were tested for the successful establishment
of the new infection when they were adults, and these aphid
lines were retested regularly to ensure the maintenance of the
new aphid-symbiont combinations. All injected lines had been
maintained in the laboratory for at least a year before being used
for experiment al assays.
Heat Shock Protocol
This assay was designed to understand symbiont dynamics after
aphids have been exposed to heat shock. Aphids were exposed
to either a single peak of high temperatures or were maintained
at a steady control temperature. The “heat shock” temperature
chosen was 38.5
◦
C, which was based on a series of pilot studies
(data not shown). Our aim was to find a temperature that had
a strong negative effect on fitness, but at whi ch approximately
half the i ndividuals in an aphid population still survived. The
aim was to explore the phenotypes of the symbionts and not to
model natural situations directly. The temperature experienced
by aphids near the ground is often considerably higher than
meteorological records and depends, for example, on aspect and
slope of the site, but it is likely that aphids are exposed to similarly
high temperatures in Northern England on hot summer d ays
(
Bennie et al., 2008; Suggitt et al., 2011).
To produce age-controlled populations of each of the five
lines, groups of young adults were placed into petri dishes (9 cm
diameter) that contained a single broad bean (Vicia faba var.
Sutton Dwarf) leaf, placed in 2% agar. V. faba is a host plant that
almost all pe a aphids perform on (
Ferrari et al., 2008). The adults
were left to reproduce for 24 h at 20
◦
C, and the offspring were
subsequently put onto 2 week old V. faba plants in groups of 50
and enclosed in a vented, transparent cage. On the following day
(aphid age 24–48 h) the populations were moved into cabinets
where they were either exposed to heat st ress or 20
◦
C as a
control. Temperature cabinets were rigorously checked before
and during the experiments to ensure even distribution of heat
and the same relative humidity (50%) in both cabinets. Plants
containing aphids were also placed in a randomized block pattern
within the cabinet to remove any potential effects of uneven
heat distribution.
While the control treatment was left at 20
◦
C, the temperature
in the heat treatment was increased from 20 to 38.5
◦
C ste adily
over the course of 2 h, held at 38.5
◦
C for 4 h, and then
decreased back to 20
◦
C over a further 2 h. Surviving aphids
from bot h treatments were moved onto fresh 2-week-old plants
on the day after heat shock to mitigate any temperature
effects on the plant itself. These plants were moved into a
different controlled-temperature room (20
◦
C), where aphids
from both heat treatments were kept together until being
collected for analysis.
Aphids were removed to measure symbiont density at two
time points. The first was 24–26 h after the start of the peak heat
shock period (when the aphids were 3 days old), and the second
11 d ays post-heat shock (when the aphids were 14 days old, ∼6
days after an aphid would usually begin reproducing). These
two time points were chosen to investigate symbiont densities
immediately after stress, and to test if recovery by t h e onset
of reproduction was possible. Buchnera densities are known to
decrease as aphids age (
Simonet et al., 2016) and so this second
time point was chosen to be the potential highest density of
Buchnera during an aphid’s development.
The aphids were flash frozen using dry ice and kept at
−80
◦
C until DNA extraction. In addition, one surviving apterous
individual from each group was placed on a petri dish with a V.
faba leaf (as above) to measure the number of offspring produced.
These dishes were refreshed every 3–4 days to ensure healt hy V.
faba leaves. Offspring counts continued until all aphids had died,
measuring total lifetime fecundity. There were 5–6 replicates
for fecundity counts and symbiont density for each of the five
aphid lines in each treatment (i.e., 10–12 replicates in total for
each line), these were performed in two temporal blocks with
approximately half the replicates of each treatment in each block.
qPCR Protocol
DNA was extracted from the aphids after samples were defrosted
at room temperature. Aphids were homogenized in a 200 µl
5% Chelex solution made in distilled water. Ten microlite rs of
proteinase K (10 mg/ml) was added per sample, and samples were
incubated for 6 h at 56
◦
C to facilitate digestion. They were then
“boiled” at 100
◦
C for 10 min before being centrifuged at 13,000
rpm for 3 min and the supernatant containing the DNA pipetted
into a clean 1.5 ml Eppendorf tube which was stored at −20
◦
C
until use. Five aphids per replicate were pooled to generate a
sample for the first time point (24–26 h after heat shock) and one
aphid for the second time point (11 days later).
Samples were run in duplicate using SYBR
R
Green reagent on
a StepOnePlus
TM
Real Time PCR machine (A pplied Biosystems).
Frontiers in Ecology and Evolution | www.frontiersin.org 3 March 2020 | Volume 8 | Article 56

Heyworth et al. Symbionts Aid Recovery From Heat-Shock
Each reaction consisted of 10 µl FAST SYBR 2× mastermix
(Applied Biosystems), 1 µl forward primer (7 µM), 1 µl reverse
primer (7 µM), 6 µl nuclease-free water, and 2 µl DNA sample.
qPCR primers for Regiella, Fukatsuia, Hamiltonella, and the
aphid housekeeping gene elongation factor-1 alpha (EF-1α)
(Supplementary Table S2) were tested to ensure high efficiency
and similarity between primer sets. Melt curves were performed
on each plate to ensure the primers were specific to each target
and only bound once. Cycling conditions were 95
◦
C for 20 s,
followed by 40 cycles of 95
◦
C for 3 s and 60
◦
C for 30 s. The
melt curve involved a further 95
◦
C for 15 s, 60
◦
C for 1 min
and then a gradual increase to 95
◦
C over 15 min. Each 96-well
qPCR plate was a nalyzed using StepOne Software v2.2.2 (Applied
Biosystems) and Cycle threshold (Ct) values were obtained by
comparing each primer sample to a single standard curve of
known concentration and using identical t h reshold and baseline
levels for each primer target across plates. Standard curves
were created by amplifying positive control samples using PCR,
calculating DNA concentrations using a High Sensitivity D NA
Assay on a 2100 Bioanalyzer system (Agilent), and then serially
diluting the sample 1:10 with distilled water to create a 5-sample
curve comprising known concentrations decreasing from 10
pmol/ml. Samples with Ct values over 30 were classed as negative,
confirmed by our negative controls. This corresponds to a copy
number of <52 per 2 µl sample for all primers, and is below the
threshold of detection. Where the difference in Ct values between
technical replicates was >1.5, the sample was either rerun or not
used in the analysis.
The standard curves were used to calculate the DNA
concentration of each sample, and this was converted into copy
numbers per 2 µl of DNA extract. To control for aphid size and
extraction quality, copy numbers for each sample were presented
relative to those of a housekeeping aphid gene as a control, giving
a ratio of symbiont copy numbers to aphid copy numbers.
Statistical Analysis
Data were analyzed using the R software v. 3.4.1 (
R Core Team,
2018). Since our core question was to test whether the three
symbiont species can provide heat shock protect ion, but not to
compare th e extent of this protection, the data were analyzed
separately for each symbiont species. Thus, in each analysis the
infected line was compared with the same uninfected aphid
genotype, within and across heat treatments. The d a ta for the
uninfected line 218 was therefore used twice, paired wit h line 218
infected with Hamiltonella or Fukatsuia. Similarly, we analyzed
the two time points separately because symbiont densities
change during aphid development, which would complicate the
interpretation of th e analysis.
Lifetime fecundity of the set of Regiella lines was analyzed
using a general linear model assuming a normal error
distribution. The number of offspring was the response
variable, and temporal block, facultative symbiont presence,
heat treatment and the interaction between symbiont presence
and heat treatment were the explanatory variables. The sets
of Fukatsuia and Hamiltonella lines were analyzed with
a non-parametric Kruskal-Wallis test, because the model
assumptions of parametric models were not met. This was
followed by Wilcoxon tests to identify differences between
specific treatments.
The densities of the symbionts were also analyzed with a
general linear model assuming a normal error distribution.
This was split into six analyses, separate for the lines relating
to each symbiont species at each time point, to simplify the
interpretation. For Buchnera densities, t he explanatory variables
were again temporal block, f acultative symbiont presence and
heat treatment as well as the interaction between the latter factors.
For the densities of the facultative symbionts, only block and
heat treatment were explanatory variables. In most cases model
assumptions were met without transforming the data, only the
Buchnera densities at the first time point in the Regiella lines were
log-transformed. For Regiella densities at the first time point and
Buchnera densities in the set of Regiella lines at the second time
point, Kruskal Wallis and Wilcoxon tests were used as described
for t h e fecundity data.
For all general linear models, post-hoc tests were only
performed when the factor or interaction was significant in
the main analysis. This was conducted using the R package
“phia” (
De Rosario-Martinez, 2015), with Holm’s correction for
multiple comparisons. All data are available as Supplementary
Material (Data Sheets 2–4).
RESULTS
Effects of Facultative Symbionts on
Fecundity After Heat Shock
We exposed aphids to a short spike of high temperature and
measured facultative and obligate symbiont densities and aphid
fitness after 1 and 11 days. As expected, heat shock decreased
the number of offspring produced in an aphid’s lifetime in all
three sets of lines [Regiella F
(1, 19)
= 103.23, P < 0.001; Fukatsuia:
W = 109, P = 0.03, Hamiltonella: W = 136.5, P = 0.001;
Figure 1]. H owever, the extent of this decrease was modified by
the presence of Regiella and Fukatsuia [Regiella, symbiont × heat
treatment: F
(1, 19)
= 5.78, P = 0.03; Fukatsuia: heat treatment in
uninfected lines W = 36, P = 0.004 and in infected lines W =
13, P = 0.48]. Fukatsuia provided the greatest protection from
heat as there was no difference in the fecundity of the infected
lines in the control and heat shock treatment, whereas there
was a greater reduction in fecundity in the uninfected aphids
than in the infected aphi ds for the Regiella lines. In contrast,
there was a similar decrease in fecundity for both uninfected and
infected Hamiltonella lines following heat shock (uninfected: W
= 36, P = 0.004; infected: W = 34.5, P = 0.008; Figure 1). At
benign temperatures, two of the symbionts also affected lifetime
fecundity: the presence of Hamiltonella increased lifetime
fecundity (W = 4, P = 0.03), whereas Fukatsuia decreased
it (W = 34, P = 0.013), and there was no difference for
Regiella (Figure 1).
Facultative Symbiont Densities After Heat
Shock
We measured the densities of the three facultative symbionts
at two time points after exposure to heat, 24–26 h and 11
Frontiers in Ecology and Evolution | www.frontiersin.org 4 March 2020 | Volume 8 | Article 56
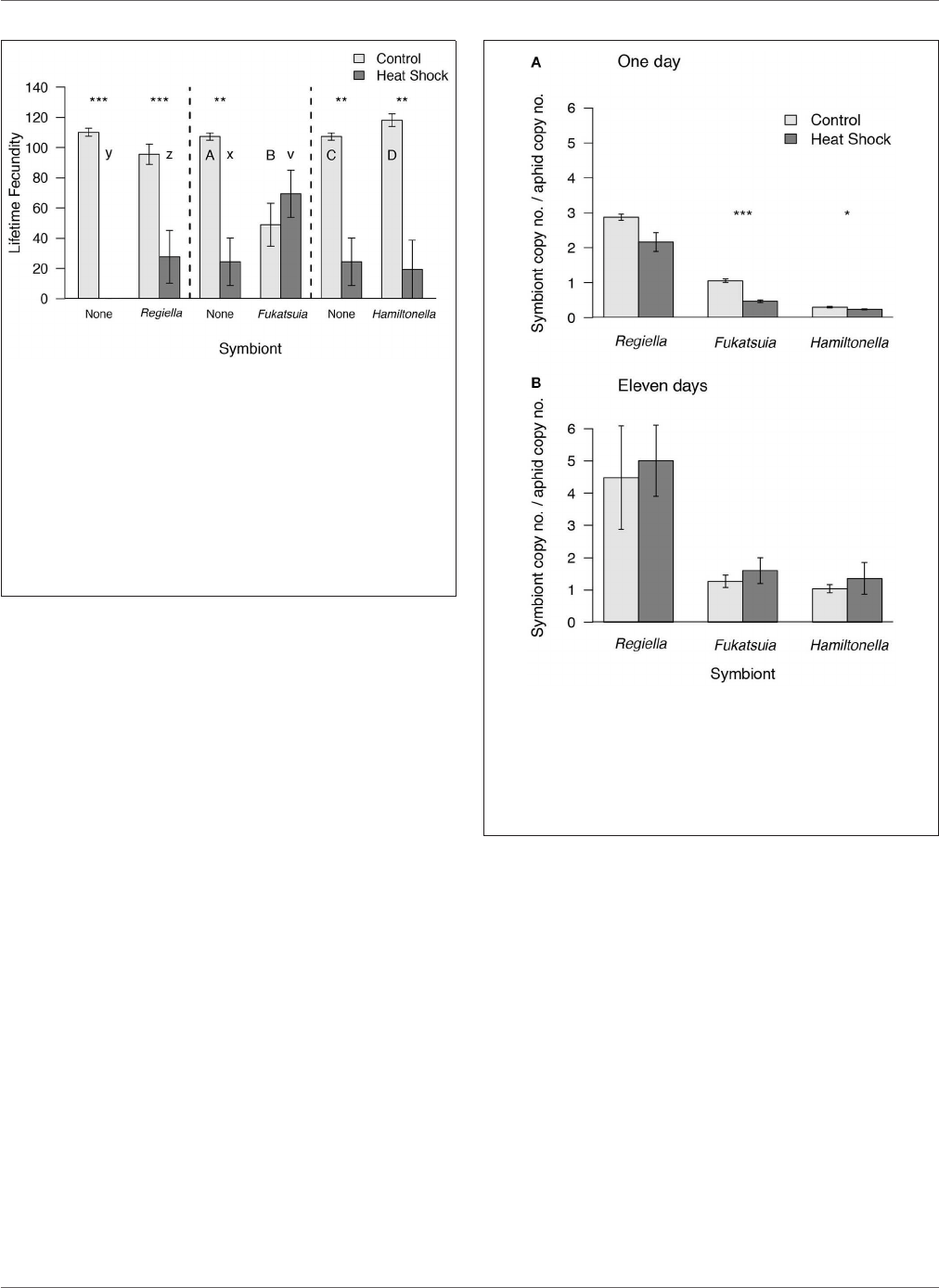
Heyworth et al. Symbionts Aid Recovery From Heat-Shock
FIGURE 1 | The effect of heat shock and facultative symbiont presence on the
number of offspring produced by pea aphids. Aphids from genotype 200 that
were uninfected or carrying Regiella are compared in the first panel. Aphids
from genotype 218 that were uninfected or carrying Fukatsuia or Hamiltonella
are compared separately in the second and third panels, respectively. In all
comparisons, there was a significant effect of heat shock compared to
controls, but no overall effect of symbiont presence. Means and standard
errors are shown. Within each panel separately, upper case letters denote
significant differences between aphids with different symbionts within the
control treatment and lower case letters denote significant differences in the
heat shock treatment. The asterisks show significant differences between heat
treatments for aphids of the same symbiont status (**P < 0.01, ***P < 0.001).
days post-heat shock (Figure 2). Compared to non-heat shocked
controls the densities of two of the symbionts, Fukatsuia and
Hamiltonella, were lower on the day after heat shock [Fukatsuia:
F
(1, 8)
= 65.05, P < 0.001, Hamiltonella: F
(1, 8)
= 6.64, P =
0.03], whereas densities of Regiella are unaffected (W = 28, p
= 0.13; but note that this is significant in a less conservative
parametric test). By the second time point, taken when the
aphids were young adults, there was no difference between
population densities in heat stressed or control aphids for any
of the three facultative symbionts [Fukatsuia: F
(1, 8)
= 0.35, P =
0.57, Hamiltonella: F
(1, 7)
= 0.95, P = 0.36, Regiella: F
(1, 6)
= 0.01,
P = 0.91; Figure 2], suggesting that heat did not have long-term
effects on faculta tive symbiont populations.
Obligate Symbiont Densities Under Heat
Shock
Compared to the control treatment densities of Buchnera were
decreased on the day after he at shock in each of the three pairs
of lines, regardless of facultative symbiont infection [Regiella
lines: F
(1, 19)
= 80.36, P < 0.001, Fukatsuia lines: F
(1, 18)
=
70.71, P < 0.001, Hamiltonella lines: F
(1, 18)
= 56.07, P <
0.001; Figure 3]. Regardless of treatment, Buchnera densities
were higher in the lines harboring Fukatsuia [F
(1, 18)
= 33.86, P <
0.001] or Hamiltonella [F
(1, 18)
= 24.52, P < 0.001] compared to
uninfected lines, an effect that was not seen in the Regiella lines
[F
(1, 19)
= 0.15, P = 0.71]. For the Fukatsuia and Hamiltonella
lines there was also a significant interaction between symbiont
presence and heat treatment [Fukatsuia: F
(1, 18)
= 9.57, P =
0.006; Hamiltonella: F
(1, 18)
= 4.80, P = 0.04]: in both cases,
FIGURE 2 | Densities of facultative symbionts in pea aphids after heat shock
or in the control treatment (A) 24–26 h after the onset of the heat shock and
(B) 11 days after heat shock. Densities are shown as the copy number of the
gyrB gene of the facultative symbiont relative to copy number of the aphid
gene EF1-α. Means and standard errors are shown. Asterisks denote
differences between heat treatments for aphids carrying a given symbiont
(*P < 0.05, ***P < 0.001).
Buchnera densities in the control treatment were higher in lines
with facultative symbionts compared to uninfected aphids but
there was no difference between these lines after heat shock. The
interaction was not significant for the Regiella lines [F
(1, 19)
=
2.58, P = 0.13] where the extent of the loss of Buchnera did not
differ between infected and uninfected lines.
At the later time point, when aphids were young adults, heat
shock again reduced Buchnera densities on average [Regiella
lines: W = 105, P = 0.004; Fukatsuia lines: F
(1, 19)
= 6.85, P =
0.02; Hamiltonella lines: F
(1, 17)
= 24.84, P < 0.001]. Fukatsuia
presence on average also significantly increased the density of
Buchnera regardless of treatment which was due to high Buchnera
densities in the heat shocked aphids [F
(1, 19)
= 7.89 , P = 0.01]; a
difference that was not found for Regiella (W = 46, P = 0.37)
or Hamiltonella presence [F
(1, 17)
= 0.17, P = 0.69]. Importantly,
there was a significant interaction between symbiont infection
and temperature in the Fukatsuia lines [F
(1, 19)
= 7.55, P = 0.01]
Frontiers in Ecology and Evolution | www.frontiersin.org 5 March 2020 | Volume 8 | Article 56

Heyworth et al. Symbionts Aid Recovery From Heat-Shock
FIGURE 3 | The effect of heat shock and facultative symbiont presence on
densities of the primary symbiont Buchnera in pea aphids. In each panel
Buchnera densities are shown 1 or 11 days after heat shock. Uninfected
aphids are compared to aphids carrying (A) Regiella, (B) Fukatsuia, and (C)
Hamiltonella. The uninfected replicates are the same for the Fukatsuia and
Hamiltonella lines. Means and standard errors are shown. In all cases, there
was a significant overall difference between the heat shock and control
treatments. There was also a significant overall effect of symbiont presence for
the Fukatsuia lines at both time points, and Hamiltonella lines 1 day after heat
shock. These main effects are not illustrated on the figure. post-hoc results
from significant interactions are denoted by upper case letters for significant
differences between aphids with different symbionts within the control
treatment, and lower-case letters denote s ignificant differences in the heat
shock treatment. The asterisks show significant post-hoc differences between
heat treatments for aphids of the same symbiont status
(**P < 0.01, ***P < 0.001).
and an equivalent effect in the Regiella lines (heat t reatment in
uninfected lines: W = 36, P = 0. 004 and in infe c ted lines: W
= 17, P = 0.42) that was not seen for the Hamiltonella lines
[F
(1, 17)
= 1.95, P = 0.18]: Buchnera densities after heat shock
were significantly reduced in uninfected aphids but not when
Fukatsuia or Regiella were present.
DISCUSSION
Our results show that different aphid symbionts can protect
the aphid from heat and help the obligate symbiont to recover
after heat shock. Infection with Regiella and Fukatsuia was
closely linked to Buchnera recovery after heat shock and led
to increased production of offspring compared t o uninfe ct ed
controls where as there was no such protection in aphids infected
with Hamiltonella. This pattern differs from other studies (
Russell
and Moran, 2006; Doremus and Oliver, 2017), which found
that Hamiltonella but not Regiella or Fukatsuia provided heat
protection. As different lines of insects and symbionts were
used in these studies, it is likely that these protective effects
are dependent on symbiont, host genotype or their interaction
and are thus not a universal feature of symbiont infection. The
prevalence of he at protection may be overestimated here since
we chose genotypes based on preliminary results.
Buchnera densities a re closely linked to aphid fitness.
Disrupting the obligate symbiosis by removing Buchnera leads
to large reductions in offspring production and often host death
(Koga et al., 2003; Akman Gündüz and Douglas, 2009) Overly
high densities of Buchnera can also lead to a reduction in
fitness (Chong and Moran, 2016), meaning that the relationship
between density of t h e symbiont and number of offspring
produced is not dire ct ly proportional. However, removal of
Buchnera, via antibiotics or heat, as also shown in our results,
generally leads to aphid sterility in the absence of facultative
symbionts (Dunb ar et al., 2007; Koga et al., 2007).
A key question that we addressed was whether the facultative
symbionts protect Buchnera from the effects of heat, and if so
whether Buchnera is dire c tly protected or its recovery facilitated.
The densities of both Buchnera and the facultative symbionts
were reduced 24 h after heat shock. In aphids carrying Fukatsuia
or Regiella, these densities returned to levels that were similar to
those in non-heat shocked controls, thus demonstrating a clear
role of the facultative symbionts in the recovery of Buchnera.
The most parsimonious interpretation of the observed pattern
is that th e facultative symbionts do not provide immediate
protection, although it is possible t hat the decline in Buchnera
DNA density occurs due to different processes in aphids with
and without facultative symbionts. It is conceivable that Buchnera
is only truly killed by heat inside the latter and growth
is merely arrested in the former and thus some immediate
protection occurs.
The patterns of obligate and facultative symbiont densities
obser ved here suggest that a different mechanism underlies the
heat protection provided by Regiella and Fukatsuia compared
to that provided by Serratia (
Montllor et al., 2002; Burke G.
et al., 2010). After heat shock, Serratia in pea aphids lyse and
this coincides with metabolomic changes (Burke G. et al., 2010).
Frontiers in Ecology and Evolution | www.frontiersin.org 6 March 2020 | Volume 8 | Article 56

Heyworth et al. Symbionts Aid Recovery From Heat-Shock
At the same time Buchnera densities are maintained at similar
levels to those at benign temperatures (Burke G. et al. , 2010). In
our experiments, in aphids with Regiella or Fukatsuia, Buchnera
densities initially decrease. This demonstrates that the protection
provided by the facultative symbionts is not instant and suggests
that the protection is probably not due to a constitutively
activated aphid stress response, but it is still possible that the
facultative symbionts prime a stress response by the aphid that
helps recovery later on.
The provision of heat-protective compounds, through either
lysis or release from a live cell, is also consistent with our
obser vations.
Burke G. et al. (2010) explored which metabolites
are affected by heat treatment when Serratia lyse. They found
three metabolic changes linked to the presence of the protective
Serratia after heat stress, one of which is a decrease in
concentration of the antioxidant indole-3-lactate, as well as two
other unidentified metabolites (
Burke G. et al., 2010). The initial
decline of Fukatsuia in our study suggest that lysis is likely
in this sy mbiont (and possibly in Regiella where the decline
is significant using less conservative statistics). However, the
protective compounds released during this process appear to act
later than in Serratia (Burke G. et al., 2010) as Buchnera also
declines initially. In some cases, facultative symbionts can replace
the function of an obligate symbiont (Koga et al., 2003, 2007)
and this could be a way of protecting the host from the effects of
heat. In our system, bot h Buchnera and the facultative symbionts
recover demonstrating that this functional replacement is not a
likely mechanism here.
Some strains of Buchnera are more resistant to heat than
others (
Dunbar et al., 2007; Moran and Yun, 2015) and it
is possible that the two aphid genotypes here carry different
Buchnera genotypes. Aphids in laboratory populations often
carry Buchnera strains with the ibpA
12
mutant allele that are
more sensitive to heat but have higher fitness (Burke, G. R. et al.,
2010). We did not sequence ibpA in the genotypes used here
since we were interested in the effects of facultative symbionts
and the Buchnera strain is the same within all our comparisons.
It is worth noting that the Fukatsuia and Hamiltonella lines
both had t h e same aphid and Buchnera genotype but only
Fukatsuia protected, suggesting that the Buchnera strain does
not bias our conclusions. However, in natural populations the
absence of facultative symbiont infections is correlated with a
higher incidence of this mutation (
Burke, G. R. et a l., 2010).
Aphids thus have two mechanisms which protect Buchnera from
heat: the absence of the heat-sensitive ibpA
12
mutant allele
and the presence of protective facultative symbionts. Facultative
symbionts appear to confer low fitness in the presence of ibpA
12
and are thus likely selected against in aphids with this mutation
(
Burke, G. R. et al., 2010). It thus suggests that in natural
populations facultative symbionts may only be able to rescue
the a phid Buchnera from heat in aph ids that carry the heat-
tolerant ibpA
13
allele a nd this may explain the scarcity of ibpA
12
in natural populations.
As well as the protective effect of Fukatsuia and Regiella,
we observed interesting changes in symbiont densities at
benign temperatures. Fukatsuia decreased fecundity, as shown
previously (
Heyworth and Ferrari, 2015; Doremus and Oliver,
2017
); the densities of Buchnera confirm that this is not due
to suppression of the obligate symbiont (Koga et al., 2003),
which has been observed for a costly infection by Rickettsia in
pea aphids (Sakurai et al., 2005). Surprisingly, infection with
Fukatsuia or Hamiltonella leads to an increase of Buchnera
population levels in younger aphids. This may benefit both the
aphid and Buchnera when facultative symbionts are present,
because additional nutrients may be required. It is possible that
either the aphid upre gulates Buchnera densities or that this strain
of Buchnera responds to the presence of facultative symbionts by
increasing its growth rate. In either case, the density of Buchnera
cells in infected aphids is comparable to uninfected aphids once
the aphids are adult.
The ability of facultative symbionts to protect obligate
nutritional symbionts from heat stress has implications for
the frequencies and spread of both the microbes and the
insects themselves. Many ph ytophagous insects rely on obligate
symbionts to provide essential nutrition, but these are often
vulnerable to ecologically stressful situations (
Bennett and
Moran, 2015; Kikuchi et al., 2016) due to severe genome
reduction during coevolution with their hosts (McCutcheon
and Moran, 2011). This genome reduction probably led to the
heat sensitivity that some facultative symbionts ameliorate, an
example of a symbiosis rescuing another symbiosis. It seems
improbable that this rescue resulted from close coevolution
due to the relatively transient nature of facultative symbiosis
infections (Smith et al., 2015).
As we and others (Montllor et a l., 2002; Burke G. et al.,
2010
) have shown, carrying certain isolates of facultative
symbionts can protect obligate symbionts from a single, short
exposure to heat, but it remains to be investigated whether
this protection is also effective under long-term or regular
exposure to extreme temperatures. The temperature-dependent
fitness effe ct s are likely to alter the frequencies of facultative
symbionts in natural populations, but will do so in concert
with other abiotic and biotic factors, including the frequencies
of heat-tolerant Buchnera strains. The symbionts’ ability to
affect interactions between host and natural enemies is also
well-documented (Hr
ˇ
cek et al., 2016). These interactions can
be affected by a change in temperature, through an effect of
temperature on the natural enemy itself (
Roux et al., 2010;
Nguyen et al., 2013) or on the interaction between host, symbiont
and natural enemy (Guay et al., 2009; Jeffs and Lewis, 2013;
Heyworth and Ferrari, 2016). In addition, both vertical and
horizontal transmission frequencies of symbionts can be affected
by temperature (Anbutsu et al., 2008; Osaka et al., 2008; Liu et al.,
2019). A combination of temperature-dependent fitness effects
and transmission dynamics is therefore a likely reason for the lack
of a clear correlation between symbiont mediated benefits seen
in laboratory experiments and symbiont frequencies observed
in the field (Oliver et al. , 2014), and probably contributes
to the geographic variation in the composition of facultative
symbiont communities (Montllor et al., 2002; Tsuchida et al.,
2002; Sepúlveda et al., 2017
). There are, however, examples where
the patterns based on laboratory experiments are observed: the
frequencies of the aphid heat-protective symbiont Serratia are
high in host populations in the warmer climes of Southern
Frontiers in Ecology and Evolution | www.frontiersin.org 7 March 2020 | Volume 8 | Article 56

Heyworth et al. Symbionts Aid Recovery From Heat-Shock
USA (Chen and Purcell, 1997; Montllor et al., 2002) a nd more
generally in arid compared to temperate regions (Henry et al.,
2013). Similarly, land temperature correlates with symbiont
prevalence in midges (Morag et al., 2012).
Facultative symbionts alter insect fitness under stressful
conditions and can affect not just the host, but also species
that the host interacts with directly and indirectly (
McLean and
Godfray, 2016; Doremus et al., 2018). In extreme cases, hosting
a defensive symbiont can lead to cascading extinctions and
the collapse of entire communities (Sanders et al., 2016). Our
work highlights how the host and its symbiont community is
affected by temperature and that this temperature-dependency
might result in changes of community interactions under
climate change.
DATA AVAILABILITY STATEMENT
All datasets generated for this study are included in the
article/Supplementary Material.
AUTHOR CONTRIBUTIONS
EH, MS, and JF conceived the ideas and designed methodology,
collected the data, wrote the manuscript, and gave final approval
for publicati on. EH and JF analyzed the data .
FUNDING
EH was supported by a studentship from th e Biotechnology
and Biological Sciences Research Council (BBSRC, award
BB/F016751/1) and JF and MS by award BB/J00524X/1
from BBSRC.
ACKNOWLEDGMENTS
We would like to thank Alison Fenwick, Chris Lancaster, and
Paul Scott for setting up and monitoring the cabinets for the heat
shock treatment, Sally James in the Technology Facility at the
University of York for support with the qPCR , and Sa lly Raines
for helping with aphid culture and helpful discussions.
SUPPLEMENTARY MATERIAL
The Supplementary Material for this article can be found
online at: https://www.frontiersin.org/articles/10.3389/fevo.
2020.00056/full#supplementary-material
Data Sheet 2 | Densities of the obligate sy mbiont Buchnera aphidicola in the two
heat treatments in the presence and absence of facultative symbionts.
Data Sheet 3 | Densities of the facultative s ymbionts in the two heat treatments.
Data Sheet 4 | Lifetime fecundity of pea aphids in the two heat treatments in the
presence and absence of facultative symbionts.
REFERENCES
Akman Gündüz, E., and Douglas, A. E. (2009). Symbiotic bacteria enable
insect to use a nutritionally inadequate diet. Proc. Biol. Sci. 276, 987 –9 91.
doi: 10.1098/rspb.2008.1476
Anbutsu, H., Goto, S., and Fukatsu, T. (2008). High and low temperatures
differently affect infection density and vertical transmission of male-killing
Spiroplasma symbionts in Drosophila hosts. Appl. Env. Microbiol. 7 4,
6053–6059. doi: 10.1128/AEM.01503-08
Bennett, G. M., and Moran, N. A. (2015). Herit able symbiosis: the advantages
and perils of an evolutionary rabbit hole. Proc. Natl. Acad. Sci. U.S.A. 112,
10169–10176. doi: 10.1073/pnas.1421388112
Bennie, J., Huntley, B., Wiltshire, A., Hill, M. O., and Baxter, R. (2008).
Slope, aspect and climate: spatially explicit and implicit models of
topographic microclimate in chalk grassland. Ecol. Model. 216, 47–59.
doi: 10.1016/j.ecolmodel.2008.04.010
Bordenstein, S. R., and Bordenstein, S. R. (2011). Temperature affects
the tripartite interactions between bacteriophage WO, Wolbachia, and
cytoplasmic incompatibility. PLoS ONE 6:e29106 . doi: 10.1371/journal.pone.
0029106
Brownlie, J. C., and Johnson, K. N. (2009). Symbiont-mediated protection
in insect hosts. Trends Microbiol. 17, 348–354. doi: 10.1016/j.tim.2009.
05.005
Brumin, M., Kontsedalov, S., and Ghanim, M. (2011). Rickettsia influences
thermotolerance in the whitefly Bemisia tabaci B biotype. Insect Sci. 18, 57–66.
doi: 10.1111/j.1744-7917.2010.01396.x
Burke, G., Fiehn, O., and Moran, N. (2010). Effects of facultative symbionts
and heat stress on the metabolome of pea aphids. ISME J. 4, 242–252.
doi: 10.1038/ismej.2009.114
Burke, G. R., McLaughlin, H. J., Simon, J. C., and Moran, N. A. (2010). Dynamics
of a recurrent Buchnera mutation that affects thermal tolerance of pea aphid
hosts. Genetics 186, 367–372. doi: 10.1534/genetics.110.117440
Carruthers, M. D., and Minion, C. (2009). Transcriptome analysis of Escherichia
coli O157:H7 EDL933 during heat shock. FEMS Microbiol. Lett. 295, 96–102.
doi: 10.1111/j.1574-6968.2009.01587.x
Chen, C. Y., Lai, C. Y., and Kuo, M. H. (2009). Temperature effect on the growth of
Buchnera endosymbiont in Aphis craccivora (Hemiptera: Aphididae). Symbiosis
49, 53–59. doi: 10.1007/s13199-009-0011-4
Chen, D. Q., and Purcell, A. H. (1997). Occurrence and transmission
of facultative endosymbionts in aphids. Curr. Microbiol. 34, 220–225.
doi: 10.1007/s002849900172
Chong, R. A., and Moran, N. A. (2016). Intraspecific genetic variation in hosts
affects regulation of obligate heritable symbionts. Proc. Natl. Acad. Sci. U.S.A.
113, 13114–13119. doi: 10.1073/pnas.1610749113
Corbin, C., Heyworth, E. R., Ferrari, J., and Hurst, G. D. D. (2017).
Heritable symbionts in a world of varying temperature. Heredity 118, 10–20.
doi: 10.1038/hdy.2016.71
De Rosario-Martinez, H. (2015). phia: post-hoc interaction analysis. R package
version 0.2-1. Available online at: https://CRAN.R-project.org/package=phia
Dion, E., Polin, S. E ., Simon, J. C., and Outreman, Y. (2011). Symbiont
infection affects aphid defensive behaviours. Biol. Lett. 7, 743–746.
doi: 10.1098/rsbl.2011.0249
Dixon, A. F. G., Kindlmann, P., Leps, J., and Holman, J. (1987). Why there are
so few species of aphids, especially in the tropics. Am. Nat. 129, 58 0 –5 92.
doi: 10.1086/284659
Doremus, M. R., and Oliver, K. M. (2017). Aphid heritable symbiont
exploits defensive mutualism. Appl. Environ. Microbiol. 83:e03276-16.
doi: 10.1128/AEM.03276-16
Doremus, M. R., Smith, A. H., Kim, K. L., Holder, A. J., Russell, J. A., and Oliver, K.
M. (20 18 ). Bre akdown of a defensive symbiosis, but not endogenous defences,
at elevated temperatures. Mol. Ecol. 27, 2138–2151. doi: 10.1111/mec.14399
Douglas, A. E. (1998). Nutritional interactions in insect-microbial symbioses:
aphids and their symbiotic bacteria Buchnera. Annu. Rev. Entomol. 43, 17–37.
doi: 10.1146/annurev.ento.43.1.17
Dunbar, H. E., Wilson, A. C. C., Ferguson, N. R., and Moran, N. A. (2007). Aphid
thermal tolerance is governed by a point mutation in bacterial symbionts. PLoS
Biol. 5: e0050096. doi: 10.1371/journal.pbio.0050096
Ferrari, J., Via, S., and Godfray, H. C. J. (2008). Population differentiation and
genetic variation in performance on eight hosts in the pea aphid complex.
Evolution 62, 2508–2524. doi: 10 .111 1 /j.1 55 8- 5 64 6.2 00 8.0 04 68 .x
Frontiers in Ecology and Evolution | www.frontiersin.org 8 March 2020 | Volume 8 | Article 56

Heyworth et al. Symbionts Aid Recovery From Heat-Shock
Ferrari, J., West, J. A., Via, S., and Godfray, H. C. J. (2012). Population genetic
structure and secondary symbionts in host-associated populations of the pea
aphid complex. Evolution 66, 375–39 0. doi: 10.1111/j.1558-5646.2011.01436.x
Gerardo, N. M., and Parker, B. J. (2014). Mechanisms of symbiont-conferred
protection against natural enemies: an ecological and evolutionary framework.
Curr. Opin. Insect Sci. 4, 8–14. doi: 10.1016/j.cois.2014.08.002
Gómez-Valero, L., Silva, F. J., Simon, J. C., and Latorre, A. (2007). Genome
reduction of the aphid endosymbiont Buchnera aphidicola in a recent
evolutionary time scale. Gene 389, 87–95. doi: 10.1016/j.gene.2006.10.001
Guay, J. F., Boudreault, S., Michaud, D., and Cloutier, C. (2009). Impact
of environmental stress on aphid clonal resistance to parasitoids: role
of Hamiltonella defensa bacterial symbiosis in association with a new
facultative symbiont of the pea aphid. J. Insect Physiol. 55, 919–926.
doi: 10.1016/j.jinsphys.2009.06.006
Harrington, R., Woiwod, I., and Sparks, T. (1999). Climate
change and trophic interactions. Trends Ecol. Evol. 14, 146–150.
doi: 10.1016/S0169-5347(99)01604-3
Henry, L. M., Peccoud, J., Simon, J. C., Hadfield, J. D., Maiden, M. J. C., Ferrari,
J., et al. (2013). Horizontally transmitted symbionts and host colonization of
ecological niches. Curr. Biol. 23, 1713–1717. doi: 10.1016/j.cub.2013.07.029
Heyworth, E. R., and Ferrari, J. (2015). A facultative endosymbiont in
aphids can provide diverse ecological benefits. J. Evol. Biol. 28, 1753–1760.
doi: 10.1111/jeb.12705
Heyworth, E. R., and Ferrari, J. (2016). Heat stress affects facultative symbiont-
mediated protection from a parasitoid wasp. PLoS ONE 11:e0167180.
doi: 10.1371/journal.pone.0167180
Hr
ˇ
cek, J., McLean, A. H. C., and Godfray, H. C. J. (2016). Symbionts modify
interactions between insects and natural enemies in the field. J. Anim. Ecol.
1605–1612. doi: 10.1111/1365-2656.12586
Jeffs, C. T., and Lewis, O. T. (2013). Effects of climate warming on host–parasitoid
interactions. Ecol. Entomol. 38, 209–218. doi: 10.1111/een.12026
Kikuchi, Y., Tada, A., Musolin, D. L., Hari, N., Hosokawa, T., Fuj isaki, K., et al.
(2016). Collapse of insect gut symbiosis under simulated climate change. mBio
7:16. doi: 10.1128/mBio.01578-16
Kim, J. K., Lee, J. B., Huh, Y. R., Jang, H. A., Kim, C. H., Yoo, J. W., et al. (2015).
Burkholderia gut symbionts enhance the innate immunity of host Riptortus
pedestris. Dev. Comp. Immunol. 53, 265–269. doi: 10.1016/j.dci.2015.07.006
Koga, R ., Tsuchida, T., and Fukatsu, T. (2003). C hanging partners in an
obligate symbiosis: a facultative endosymbiont can compensate for loss of the
essential endosymbiont Buchnera in an aphid. Proc. Biol. Sci. 270, 2543–2 55 0.
doi: 10.1098/rspb.2003.2537
Koga, R., Tsuchida, T., Sakurai, M., and Fukatsu, T. (2007). Selective
elimination of aphid endosymbionts: effects of antibiotic dose and host
genotype, and fitness consequences. FEMS Microbiol. Ecol. 60, 229–239.
doi: 10.1111/j.1574-6941.2007.00284.x
Kwong, W. K., Mancenido, A. L., and Moran, N. A. (2017). Immune system
stimulation by the native gut microbiota of honey bees. R. Soc. Open Sci.
4:170003. doi: 10.1098/rsos.170003
Laughton, A. M., Fan, M. H., and Gerardo, N. M. (2013). The combined
effects of bacterial symbionts and ageing on life history traits in the
pea aphid Acyrthosiphon pisum. Appl. Environ. Microbiol. 80, 470–477.
doi: 10.1128/AEM.02657-13
Liu, X. D., Lei, H. X., and Chen, F. F. (2019). Infection pattern and negative effects
of a facultative endosymbiont on its insect host are environment-dependent.
Sci. Rep. 9:4013. doi: 10.1038/s41598-019-40607-5
Maire, J., Vincent-Monégat, C., Masson, F., Zaidman-Rémy, A., and Heddi,
A. (2018). An IMD-like pathway mediates both endosymbiont control
and host immunity in the cereal weevil Sitophilus spp. Microbiome 6:6.
doi: 10.1186/s40168-017-0397-9
Martinez, J., Ok, S., Smith, S., Snoeck, K., Day, J. P., and Jiggins, F. M.
(2015). Should symbionts be nice or selfish? antiviral effects of Wolbachia
are costly but reproductive parasitism is not. PLoS Pathog. 11:e1005021.
doi: 10.1371/journal.ppat.1005021
McCutcheon, J. P., and Moran, N. A. (2011). Extreme genome reduction in
symbiotic bacteria. Nat. Rev. Microbiol. 10, 13–26. doi: 10.1038/nrmicro2670
McLean, A., Van Asch, M., Ferrari, J., and Godfray, H. C. J. (2011). Effects of
bacterial secondary symbionts on host plant use in pea aphids. Proc. Biol. Sci.
278, 760–766. doi: 10.1098/rspb.2010.1654
McLean, A. H. C., and Godfray, H. C. J. (2016). The outcome of competition
between two parasitoid species is influenced by a facultative symbiont of their
aphid host. Funct. Ecol. 31, 927–933. doi: 10.1111/1365-2435.12781
Montllor, C. B., Maxmen, A., and Purcell, A. H. (2002). Facultative bacterial
endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol.
Entomol. 27, 189–195. doi: 10.1046/j.1365-2311.2002.00393.x
Morag, N., Kle ment , E., Saroya, Y., Lensky, I., and Gottlieb, Y. (2012). Prevalence
of the symbiont Cardinium in Culicoides (Diptera: Ceratopogonidae) vector
species is associated with land surface temperature. FASEB J. 26, 4025–4034.
doi: 10.1096/fj.12-210419
Moran, N. A. (1996). Accelerated evolution and Muller’s ratchet in
endosymbiotic bacteria. Proc. Natl. Acad. Sci. U.S.A. 93, 2873–2878.
doi: 10.1073/pnas.93.7.2873
Moran, N. A., and Yun, Y. (2015). Experimental replacement of an
obligate insect symbiont. Proc. Natl. Acad. Sci. U.S.A. 112, 2093–2096.
doi: 10.1073/pnas.1420037112
Neelakanta, G., Sultana, H., Fish, D., Anderson, J. F., and Fikrig, E. (2010).
Anaplasma phagocytophilum induces Ixodes scapularis ticks to express an
antifreeze glycoprotein gene that enhances their survival in the cold. J. Clin.
Invest. 120, 3179–3190. doi: 10.1172/JCI42868
Nguyen, T. M., Bressac, C., and Chevrier, C. (2013). Heat stress affects
male reproduction in a parasitoid wasp. J. Insect Physiol. 59, 248–254.
doi: 10.1016/j.jinsphys.2012.12.001
Oliver, K. M., Moran, N. A., and Hunter, M. S. (20 05 ). Variation in resistance to
parasitism in aphids is due to symbionts not host genotype. Proc. Natl. Acad.
Sci. U.S.A. 102, 12795–12800. doi: 10.1073/pnas.0506131102
Oliver, K. M., Smith, A. H., and Russell, J. A. (2014). Defensive symbiosis in the real
world – advancing ecological studies of heritable, protective bacteria in aphids
and beyond. Funct. Ecol. 28, 341–355. doi: 10.1111/1365-2435.12133
Osaka, R., Nomura, M., Watada, M., and Kageyama, D. (2008). Negative effects
of low temperatures on the vertical transmission and infection density of a
Spiroplasma endosymbiont in Drosophila hydei. Curr. Microbiol. 57, 335–339.
doi: 10.1007/s00284-008-9199-4
Parmesan, C., and Yohe, G. (2003). A globally coherent fingerprint of
climate change impacts across natural systems. Nature 421, 37–42.
doi: 10.1038/nature01286
R Core Team (2018). R: A Language and Environment for Statistical Computing.
Vienna: R Foundation for Statistical Computing. Available online at: http://
www.R-project.org/ (accessed April 9, 2019).
Roux, O., Le Lann, C., van Alphen, J. J. M., and van Baaren, J. (2010). How does
heat shock affect the life history traits of adults and progeny of the aphid
parasitoid Aphidius avenae (Hymenoptera: Aphidiidae)? Bull. Entomol. Res.
100, 543–549. doi: 10.1017/S0007485309990575
Russell, J. A., and Moran, N. A. (2006). Costs and benefits of symbiont infection
in aphids: variation among symbionts and across temperatures. Proc. Biol. Sci.
273, 603–610. doi: 10.1098/rspb.2005.3348
Sakurai, M., Koga, R., Tsuchida, T., Meng, X. Y., and Fukatsu, T. (2005). Rickettsia
symbiont in the pea aphid Acyrthosiphon pisum: novel cellular tropism, effect
on host fitness, and interaction with the essential symbiont Buchnera. Appl.
Environ. Microbiol. 71, 4069–4075. doi: 10.1128/AEM.71.7.4069-4075.2005
Sanders, D., Kehoe, R., van Veen, F. J. F., McLean, A., Godfray, H. C. J., Dicke,
M., et al. (2016). Defensive insect symbiont leads to cascading extinctions and
community collapse. Ecol. Lett. 19, 789–799. doi: 10.1111/ele.12616
Schmitz, A., Anselme, C., Ravallec, M., Rebuf, C., Simon, J. C., Gatti, J. L., et al.
(2012). The cellular immune response of the pea aphid to foreign intrusion and
symbiotic challenge. PLoS ONE 7:e42114. doi: 10.1371/journal.pone.0042114
Schmitz, O. J., and Barton, B. T. (2014). Climate change effects on
behavioral and physiological ecology of predator–prey interactions:
implications for conservation biological control. Biol. Control 75, 87–96.
doi: 10.1016/j.biocontrol.2013.10.001
Sepúlveda, D. A., Zepeda-Paulo, F., Ramírez, C. C., Lavandero, B., and Figueroa,
C. C. (2017). Diversity, frequency, and geographic distribution of facultative
bacterial endosymbionts in introduced aphid pests. Insect Sci. 24, 511–521.
doi: 10.1111/1744-7917.12313
Shan, H. W., Deng, W. H., Luan, J. B., Zhang, M. J., Zhang, Z., Liu, S. S.,
et al. (2017). Thermal sensitivity of bacteriocytes constrains the persistence
of intracellular bacteria in whitefly symbiosis under heat stress. Environ.
Microbiol. Rep. 9, 706–71 6. doi: 10.1111/1758-2229.12554
Frontiers in Ecology and Evolution | www.frontiersin.org 9 March 2020 | Volume 8 | Article 56

Heyworth et al. Symbionts Aid Recovery From Heat-Shock
Simonet, P., Duport, G., Gaget, K., Weiss-Gayet, M., Colella, S., Febvay, G., et al.
(2016). Direct flow cytometry measurements reveal a fine-tuning of symbiotic
cell dynamics according to the host developmental needs in aphid symbiosis.
Sci. Rep. 6:19967. doi: 10.1038/srep19967
Smith, A. H., Łukasik, P., O’Connor, M. P., Lee, A., Mayo, G., Drott, M. T.,
et al. (2015). Patterns, causes and consequences of defensive microbiome
dynamics across multiple scales. Mol. Ecol. 24, 1135–1149. doi: 10.1111/mec.
13095
Suggitt, A. J., Gillingham, P. K., Hill, J. K., Huntley, B., Kunin, W. E.,
Roy, D. B., et al. (2011). Habitat microclimates drive fine-scale variation
in extreme temperatures. Oikos 120, 1–8. doi: 10.1111/j.1600-0706.2010.
18270.x
Tsuchida, T., Koga, R., Horikawa, M., Tsunoda, T., Maoka, T., Matsumoto, S.,
et al. (2010). Symbiotic bacterium modifies aphid body color. Science 330,
1102–1104. doi: 10.1126/science.1195463
Tsuchida, T., Koga, R., Shibao, H., Matsumoto, T., and Fukatsu, T. (2002 ). Diversity
and geographic distribution of secondary endosymbiotic bacteria in natural
populations of the pea aphid, Acyrthosiphon pisum. Mol. Ecol. 11, 2123–2135.
doi: 10.1046/j.1365-294X.2002.01606.x
Vorburger, C. (2014). The evolutionary ecology of symbiont-conferred resistance
to parasitoids in aphids. Insect Sci. 21, 251–264. doi: 10.1111/1744-7917.
12067
Walther, G. R., Post, E., Convey, P., Menzel, A., Parmesan, C., Beebee, T., et al.
(2002). Ecological responses to recent climate change. Nature 416, 38 9– 39 5.
doi: 10.1038/416389a
Weiss, B. L., Maltz, M., and Aksoy, S. (2012). Obligate symbionts activate
immune system development in the tsetse fly. J. Immunol. 188, 33 95 –3 4 03.
doi: 10.4049/jimmunol.1103691
Wilcox, J. L., Dunbar, H. E., Wolfinger, R. D., and Moran, N. A.
(2003). Consequences of reductive evolution for gene expression
in an obligate endosymbiont. Mol. Microbiol. 48, 1491–1500.
doi: 10.1046/j.1365-2958.2003.03522.x
Young, D., Roman, E., Moreno, C., O’Brien, R., Born, W., Welch, W. J., et al.
(1993). Molecular chaperones and the immune response. Philos. Trans. R. Soc.
Lond. B. Biol. Sci. 339, 363–368. doi: 10.1098/rstb.1993.0035
Zhang, B., Leonard, S. P., Li, Y., and Moran, N. A. (2019). Obligate bacterial
endosymbionts limit thermal tolerance of insect host species. Proc. Natl. Acad.
Sci. U.S.A. 116, 24712–24718. doi: 10.1073/pnas.1915307116
Conflict of Interest: The authors declare that t he research was conducted in the
absence of any commercial or financial relationships that could be construed as a
potential conflict of interest.
Copyright © 2020 Heyworth, Smee and Ferrari. This is an open-access article
distributed under the terms of the Creative Commons Attribution License (CC BY).
The use, distribution or reproduction in other forums is permitted, provided the
original author(s) and the copyright owner(s) are credited and that the original
publication in this journal is cited, in accordance with accepted academic practice.
No use, distribution or reproduction is permitted which does not comply with these
terms.
Frontiers in Ecology and Evolution | www.frontiersin.org 10 March 2020 | Volume 8 | Article 56
