
MOHAMMAD
R.
SHOJA*
MOHAMMAD
R.
BEHSHARATY*
SUMMARY: The purpose of this study is to compare the efficacy of Ketotifen fumarate 0.025% (Zaditen)
with Cromolyn sodium 4% eye drops in prevention of itching, tearing and redness in vernal keratoconjunctivitis
(VKC).
This double masked randomized single center clinical trial conducted between April and August 2004 in
Yazd. One hundred eligible patients with clinical diagnosis of moderate VKC were randomly assigned to Zaditen
(Group A, n=50) and Cromolyn sodium (Group B, n=50) eye drops for a 4 week period. Itching, lacrimation,
redness and photophobia were scored on a 4-point severity scale.
At the follow up visits, the responder rate based on subjects assessment of global efficacy was significantly
greater in Ketotifen group (71.5%) than in Cromolyn group (53%). A clear response to treatment occurred in
94.4% of Zaditen patients and 81.2% of sodium Cromoglycate patients. The investigator's assessment of respon-
der rates also showed that Ketotifen was superior to Cromolyn sodium (p=0.001). Ketotifen produced a signif-
icantly better outcome than Cromolyn (p<0.05) for relief of signs and symptoms of VKC. Ketotifen fumarate
treatment significantly reduced the total signs and symptoms score for each patient compared to day 0.
Ketotifen had a faster onset of action and provided better symptom relief than Cromolyn: the rapid onset of
action and symptom control, make Zaditen a valuable treatment for VKC.
Key words : VKC, Allergic conjunctivitis, Zaditen.
Ophthalmology
INTRODUCTION
Vernal keratoconjunctivitis (VKC) is a bilateral ocular
allergic disease tending to occur in children during spring
and summer months (1). The disease occurs in warm
temperate zone and is more common in the Middle East,
Mediterranean area (2), and Iran.
VKC can certainly pose a threat to vision due to
corneal involvement (3).
The immunopathogenesis appears to involve both
types I and IV hypersensitivity (4, 5). The treatment of
VKC is quite prolonged and demands good compliance.
Presently moderate to severe cases were treated
with mast-cell stabilizers such as Cromolyn sodium and
topical corticosteroids (6).
However, the risks of prolonged use of corticos-
teroids are cataract and glaucoma, and should be
reserved for treatment of severe eye symptoms. In VKC
mast cell degranulation and release of histamine stimulate
* From Department of Ophthalmology, Shahid Sadughi School of
Medicine, Yazd, Iran.
CCOOMMPPAARRIISSOONN
OOFF
EEFFFFIICCAACCYY
AANNDD
SSAAFFEETTYY
OOFF
TTOOPPIICCAALL
KKEETTOOTTIIFFEENN
((ZZAADDIITTEENN))
AANNDD
CCRROOMMOOLLYYNN
SSOODDIIUUMM
IINN
VVEERRNNAALL
KKEERRAATTOOCCOONNJJUUNNCCTTIIVVIITTIISS
35Medical Journal of Islamic World Academy of Sciences 16:1, 35-40, 2006
Medical Journal of Islamic World Academy of Sciences 16:1, 35-40, 200636
COMPARISON OF ZADITEN WITH CROMOLYN SHOJA, BEHSHARATY
day 7 and 15. Responder rates were also assessed at the termi-
nation visit held at day 30.
Ocular status assessment
Different symptoms (itching, tearing, burning, redness) and
signs (watery, discharge increase, swelling, presence of follicles)
of VKC were evaluated at their enrollment (day zero) and at dif-
ferent times after starting treatment (7, 15 and 30 days). Symp-
toms and signs were classified in four stages: 0- Absent, 1- Mild,
2- Moderate and 3- Severe. The total symptoms and signs score
(TSSS) for each subject were obtained by adding the values of
each symptoms and signs divided by the total number of them.
Each patient was instructed to grade his or her symptoms of itch-
ing, photophia, watering, and mucoidal discharge on a scale from
0 to 3. The patients scored their symptoms for both eyes. Clinical
signs (conjunctival erythema, conjunctival chemosis, papillae,
limbal hypertrophy, presence of follicles) were also collected from
the right eye of each patient at the beginning, follow-up and at the
end of the study. Each patient was examined and clinically
scored by an ophthalmologist who did not know either clinical
status in the pre or post treatment period or the groups of patients
(A or B). Subjects were asked to assess the overall effect of treat-
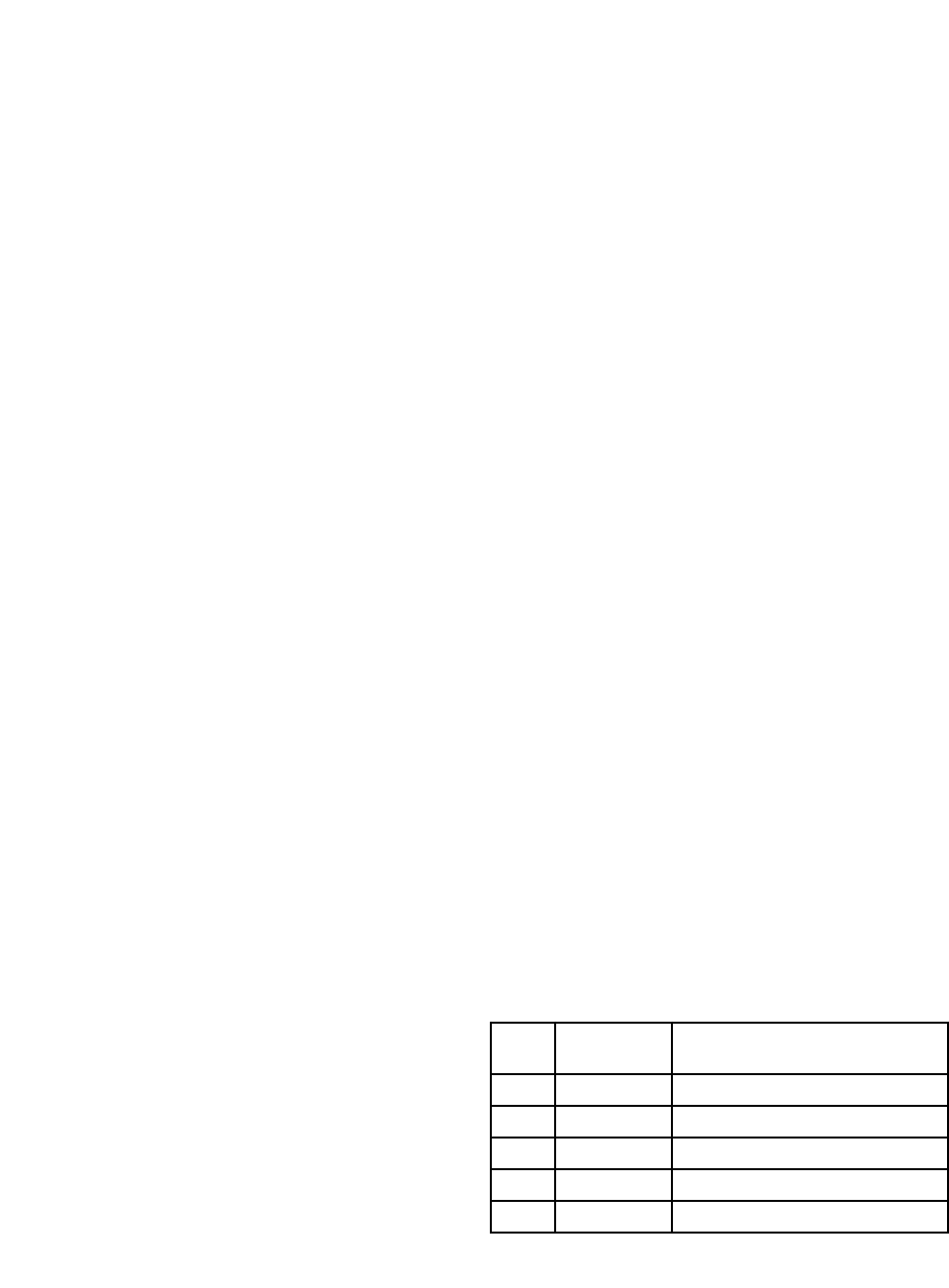
ment using a five point grading scale (Table 1).
Tolerability and safety
Assessment of tolerability was based on adverse data
obtained by the subject volunteering the information and by the
physician. At the end of the treatment investigator provided a
global assessment of safety and tolerability using the same 5-
point scale as efficacy (Table 1).
Statistical analysis
The Kaplan-Meier technique was used to describe the onset
time distributions of the two treatments, with the planned observa-
tion time intervals. The onset time distributions were compared
between the two treatment groups using a long-rank test and a
general linear means model. The long-rank test was used to
check the primary efficacy variable. This test is most sensitive to
postpone the responder rate, signs and symptoms were analyzed
using logistic regression for binary and ordinary data respectively.
nerve endings and leading to dilation of the blood vessels
causing itching and redness (7). Mast cell stabilizing by
Cromolyn has an important role for treatment. More
recently interest has focused on the possibility of topical
application of histamine H1-antagonists.
Ketotifen a benzocycloheptathiophene derivate has
been used in the treatment of asthma (8). It blocks hista-
mine H1 receptors, stabilizes mast cells and prevents
eosinophil accumulation and degranulation (9, 10).
Ketotifen fumarate 0.025% ophthalmic solution
(Zaditen) has been developed recently for alleviating the
ocular signs and symptoms of VKC (11). Recent clinical
trials demonstrated that Zaditen 0.025% eye drop was
efficacious and safe, providing a rapid onset and long
duration of action (12-14).
The purpose of this study was to compare the effi-
cacy and safety of ketotifen fumarate 0.025% ophthalmic
solution (Zaditen) with Cromolyn sodium 4% in the treat-
ment of moderate VKC.
MATERIALS AND METHODS
Subjects
This study was performed between April and August 2004
in Yazd province. One hundred subjects (68 males and 32
females ) enrolled in this research.
Subjects eligible for inclusion were required to be between
age of 8 and 25 years and suffering from moderate VKC, and all
had papillae on the upper tarsal conjunctiva, conjunctival ery-
thema, limbal hypertrophy and typical mucoid discharge. Sub-
jects with history of dry eye, other form of allergic conjunctivitis,
allergy to antihistamines, the ocular surgery within 2 months
before study and who had systemic or ocular corticosteroids or
mast cell slabitisers within 4 weeks of randomization were
excluded. Patients’ written informed consent was required. The
trial was conducted in accordance with the declaration of Helsinki
prior to enrolment as study design.
Study design
This was single center double - masked randomized com-
parative clinical trial, and the patients were randomly divided into
two equal groups (A and B).
Group A patients (n=50) received topical Zaditen 0.025%
twice a day and placebo one time a day. Topical Cromolyn
sodium 4% was prescribed to Group B (n=50) three times a day.
Each group contained 34 males and 16 females.
Treatment was given to each group for 4 weeks, the pack-
aging of all trial medications was identical in appearance. The
study involved three visits, a screening visit and two treatment
visits. Primary analysis was at the follow up visit held between
Table 1: Subject and investigator assessment of global efficacy
relative to baseline.
Score
Change
from baseline
Description
0 Excellent Complete relief of ocular symptoms
1 Good Distinct relief of ocular symptoms
2 Fair Some relief from ocular symptoms
3 Poor No relief from ocular symptoms
4 Deterioration Worsening of ocular symptoms

Medical Journal of Islamic World Academy of Sciences 16:1, 35-40, 2006
COMPARISON OF ZADITEN WITH CROMOLYN SHOJA, BEHSHARATY
37
RESULTS
One hundred subjects were screened (68 males, 32
females). The homogeneity of treatment groups was
checked with regard to age, sex and baseline sum score.
No significant difference was noted. Study participants
were between the ages of 8 and 25 years (mean 16.3)
and had a duration of disease ranging from 1 to 15 years
(median duration 8.3 years).
Primary efficacy variable
The primary efficacy variables were a physicians
clinical judgement scale and patients overall judgement
scale of improvement from baseline.
The median time to onset of action was 15 minutes
for Zaditen versus 45 minutes for Cromolyn. Onset of
action was defined at first time interval in which at least a
20% decrease in composite ocular symptom score
occurred. At each post-dose time point, more subjects
receiving Zaditen had 20% or more reduction in symp-
toms than those receiving Cromolyn. Analyses of the time
to onset distribution (Figure 1) showed Zaditen to be sta-
tistically superior to Cromolyn (p=0.028).
Both primary efficacy variables showed significantly
greater overall improvement of VKC from baseline with
Zaditen than Cromolyn.
Responder rate
Responders were patients whose sum score of three
main eye symptoms decreased by at least 3 points from a
baseline score. After 7 days of treatment 59% of Zaditen
treated patients and 53% of Cromolyn treated patients
showed improvements of their symptoms and signs. With
continued treatment through day 14 symptoms control
was achieved in 81% of Group A and 63% of Group B and
this difference was significant (p<0.001).
At the final visit the responder rate as judged by the
subject was significantly greater with Zaditen compared to
Cromolyn (p=0.01).
Moreover administration of Zaditen eye drop for thirty
days significantly (p<0.0001) reduced the TSSS for each
patient between days 0 and 30.
A clear response to treatment (an improvement of
sum scores of ≥ 3 points compared to base line) occurred
in 94.4% of Zaditen treated patients and 81.2% of Cro-
molyn patients. Based on subject daily records the supe-
riority of Zaditen in relieving signs and symptoms
including redness and tearing was observed from the
beginning of the treatment and was most marked during
Figure 1: Onset of action by treatment groups.
Ketotifen (n=50) Cromolyn (n=50)
15
30
45 60 90
Time after drug administration (min)
100
90
80
70
60
50
40
30
20
10
0
Table 2: Mean ocular sign and symptoms at day 5-8 visit.
Mean Score P value
Signs and symptoms Zaditen (n=50) Cromolyn (n=50) Zaditen V Cromolyn
Redness 0.68 0.90 0.03
Itching 1.25 1.44 0.27
Tearing 0.53 0.88 0.01
Lid swelling 0.40 0.43 0.85
Discharge 0.12 0.24 0.82
Composite score 2.98 3.89 0.02
Subjects with
20% symptom reduction (%)

Medical Journal of Islamic World Academy of Sciences 16:1, 35-40, 200638
COMPARISON OF ZADITEN WITH CROMOLYN SHOJA, BEHSHARATY
the first day. Zaditen was superior to Cromolyn in prevent-
ing itching (p<0.001) and redness (p<0.005) at most
assessments. Mean scores for eyelid swelling and mucous
discharge were generally low for Zaditen Group (Table 2).
At the termination visit the analysis showed signifi-
cantly better relief of signs and symptoms with Zaditen
than Cromolyn (p=0.0), with mean composite sign and
symptom score of 2.98 and 3.89, respectively (Table 2).
Analysis of the mean composite ocular symptoms scores
versus time showed Zaditen to have a faster onset of
action in the relief of ocular symptoms (2 hours post-dose)
than Cromolyn sodium (Figure 2). At the end of treatment
global assessment of efficacy by the investigator was con-
sidered at least 91.4% for Zaditen and 78% for Cromolyn
sodium.
Safety
Both treatments were generally well tolerated and
majority of adverse events were of mild transient irritation
and burning. However, the droupout rate due to adverse
events was lower in the Zaditen Group (n=2.4%) com-
pared to Cromolyn (n = 4.8%).
Investigator global assessment of tolerability gave an
opinion of at least satisfactory in 95.6% of Zaditen -and
86.3% of Cromolyn sodium, treated patients.
DISCUSSION
VKC is a common, prevalent and clinically significant
IgE mediated hypersensivity response. VKC is an
immunopathological disease in which the number of mast
cells in substantia propria increase (15-16). Activation of
mast cells by IgE bound receptor crosslinking by allergen
promotes the release of several mediators such as hista-
mine, prostaglandins and cytokinase, all of which con-
tribute to the symptoms of VKC (17,18). The mast cell is
considered to play a pivotal role in producing symptoms
and signs of VKC (19). Current therapy of VKC focuses on
modulation of the immune system and pharmacologic
inhibition of the chemical mediators involved in the
immune response. Mast cell stabilizers and antihista-
mines are two of the most commonly used groups of ther-
apeutic agents. They stabilize the mast cell membranes
by preventing calcium influx across the mast cell mem-
branes, thereby preventing mast cell degranulation and
mediator release. The new antihistamines have been
demonstrated to be capable of affecting several phen-
emonea of the allergic inflammation including mediator
release (20,21).
Among these drugs, new multiple - action agents like
Ketotifen fumarate (Zaditen) is histamine H1- receptor
antagonist, as well as mast cell stabilizer.
In addition,
in vitro
and animal studies (22) have
shown that Zaditen inhibits the activation and chemotaxis
of eosinophils into the conjunctiva, (23) which is an impor-
tant step in the late phase of the immune response.
Cromolyn sodium as a mast cell stabilizer is effective
and safe in the treatment of VKC, but topical steroids are
often required which increase the chance of bacterial ker-
atitis, cataract and glaucoma, so we decided to perform a
randomized double blind study in order to investigate and
compare the effect of the topical Ketotifen with Cromolyn
sodium in modarate VKC.
Figure 2: Change in mean composite ocular symptom scores over time.
10
8
6
4
2
0
123456789End
Time (h)
Ketotifen Cromolyn
Ocular symptoms
(mean sum of scores)

Medical Journal of Islamic World Academy of Sciences 16:1, 35-40, 2006
COMPARISON OF ZADITEN WITH CROMOLYN SHOJA, BEHSHARATY
In the present study main VKC symptoms decreased
significantly by day 3 with sustained improvement on days
7 and 14.
The results of this study showed that Zaditen 0.025%
applied topically twice a day was superior to Cromolyn
QID (p=0.001). Zaditen produced a significantly better
outcome than Cromolyn (p < 0.05) for relief of signs and
symptoms of VKC. Leonardi’s study (24) showed investi-
gators assessment of responder rates for Zaditen was
superior to Cromolyn that is similar to our study. A recent
study by Andrea (25) reported a clear response of 91.2%
for Zaditen and 83.5% for Cromolyn treatment groups that
was similar to our study.
In the current study as Friedrich Horak’s (12) report
Zaditen was found to have a faster onset of action than
Cromolyn. In term of efficacy, Zaditen was numerically
superior to Cromolyn for the majority of the individual
symptoms score (26).
We can conclude that at 15 minute and 4-hour
Zaditen was superior to Cromolyn in preventing itching and
redness which was the same as Greiner’s results (27).
In this study the responder after 7 days of treatment
was 59% for Zaditen and 53% for Cromolyn treated
patients, however, in Kidd's report (28) these were 56.5%
and 49.3% respectively. In this study at the follow up visit
the responder rate based on subject’s assessment global
efficacy was significantly greater in Zaditen group (71.5%)
than in Cromolyn group (51%). That was not comparible
with Kidd's study with responder rate of 49.5% and 33%
respectively.
CONCLUSION
Zaditen 2 times a day was significantly more effec-
tive than sodium Cromolyn four times a day in alleviating
symptoms and signs of moderate VKC. The faster onset
action (within 15 minutes) and better symptoms relief
observed with Ketotifen during the initial 2 hours, along
with favourable safety and tolerability profile make Zaditen
a new valuable treatment option for patients with moder-
ate VKC.
REFERENCES
1. Allansmetl MR, Intasman W, Jaeger EA : Duane's clinical
ophthalmology, revised. Philadelphia Lippincott, Vernal conjunc-
tivitis, vol 5, chap 9, 1995.
2. Buckley RY : Vernal keratoconjunctivitis (review). Int Oph-
thalmol Clin, 66:112-117, 1998.
3. Cameron J : A shield ulcers and plaques of the cornea in
vernal keratoconjunctivitis. Ophthalmology, 102:985-993, 1995.
4. Borini S, Bonini S : JgE and non - JgE mechanisms in
ocular allergy. Aum Allergy, 11-296-299, 1993.
5. Foster CS : Atopic keratoconjunctivitis. Ophthalmology,
99: 992-1000, 1990.
6. Abelson MB, George MA, Smilt LM : Evaluation of 0.05%
levocabostine versus 4% cromolyn in the allergen challange
model. Ophthalmology, 102:310-316, 1995.
7. Leonardi A : Role of Histamine in allergic conjunctivitis.
Acta Ophthalmol Scand, 230: 18-21, 2000.
8. Cartier A, Bernstein IL, Burge PS, et al : Guidelines for
bronchoprovocation on the investigation of occupational asthma.
Report of the subcommittee, on Bronchoprovocation for occupa-
tional Asthma. J Allergy Clin Immunol, 84:823-829, 1989.
9. Grant SM, Goal KL, Fitton A, et al : Ketotifen: A review of
its pharmacodynamic and pharmacokinetic properties, and thera-
peutic use in asthma and allergic disorders Drugs, 40:412-448,
1999.
10. Nabe M, Miyagawa H, Agrawal DK, et al : The effect of
ketotifen on eosinophils as measured at LTC4 release and by
chlemotaxis. Allergy proc, 12:267-271, 1994.
11. Fsadim MG, Lanz R, Taylor HR, et al : Efficacy and
safety of ketotifen fumarate eye drops versus vihicle placebo and
levocabastino in an environmental. Study of patients with sea-
sonal allergic conjunctivitis (abstract): Invest Ophthalmol Vis Sci,
41:S368, 2000.
12. Friedrich Horak, Petra Stbner : Onset and duration of
action of ketotifen 0.025% and Emedastine 4% in (SAC) Clin Drug
Invest, 23:329-337, 2000.
13. D'Arianzo PA, Heonardi A, Benscl G : Randomized
double - masked, placebo - controlled comparison of the efficacy
of emedastine difurmarete 0.05% ophthalmic Solution and keto-
tifen fumarate 0.025% ophthalmic solution in the human conjunc-
tival Allergen challenge model Clin Ther, 24:409-16, 2002.
14. Gomes PJ, Welch DL, Abelson MB : Evaluation of the
efficacy and safety of ketotifen in the allergen challenge model.
Eur Jophthalmol, 13:128-33, 2003.
15. Macleod JD, Anderson DF, Baddeley SM, Holgate ST,
McGill JF, Roch WR : Immunolocalization of cytokines to mast cell
in normal and allergic conjunctiva. Clin EXP Allergy, 27:1328-
1334, 1997.
16. Irani AM : Ocular mast cells and mediators in ocular
allergy. Immunology and allergy clinics of North America (Edited
by Bielory), WB Sounders, 1-13, 1997.
17. MacDonal SM : Histamine-releasing factor. Curr Opin
Immunol, 8:778-783, 1996.
18. Desreumaux P, Capron M : Eosinophils in allergic reac-
tions. Curr Opin Immunol, 8:790-795, 1996.
19. Anderson DF, Macleod JD, Baddeley SM, Bacon AS,
39

Medical Journal of Islamic World Academy of Sciences 16:1, 35-40, 200640
COMPARISON OF ZADITEN WITH CROMOLYN SHOJA, BEHSHARATY
McGill JF, Holgate ST, Roche WR : Seasonal allergic conjunctivi-
tis is accompanied by increase mast cell numbers in the absence
of leukocyte infiltration. Clin EXP Allergy, 27:1060-1066, 1997.
20. Abelson MB, Schaeferk : Conjunctivitis of allergic origin:
immunologic mechanims and current approaches to therapy. Surv
Ophthalmol, 38:115-127, 1993.
21. Ciprandi G, Passalacqua G, Canonica GW : Effects of HI
antihistamines on adhesion molecules: a possible rationale for
long term treatment. Clin EXP Allergy, 29:49-53, 1999.
22. Sanjar S, Aoki S, Boubekeur K, et al : Inhibition of PAF-
induced eosinophil acculation in pulmonary airways of guinea pigs
by anti-asthma drugs. Jpn J Pharmacology, 51:167-172, 1989.
23. Arnoux B, Denjean A, Page CP, et al : Accumulation of
platelets and eosinophils in baboon iung after paf-acetger chal-
lenge inhibition by ketotifen. Am Rev Respir Dis, 137:855-60,
1988.
24. Leonardi A, Busca F, Tavolate M, Secchi AG : The anti-
allergic effects of a chlorphiniramine sodium-chlorp combination
compared to ketotifen in the conjuctiva challege model.
25. Andrea P, Martin Juliourrets-Zevqlia : The effect of keto-
tifen on inflammatory markers in allergic conjunctivitis. BMC oph-
thalmology, 303, pp 1-8, 2003.
26. Greiner JV, Minno GA : Placebo-controlled comparison
of ketotifen fumarate and ncdocromil sodium for prevention of
ocular ilching in VKC Clin Ther, 25:1988-2005, 2003.
27. Greiner JV, Michaelson C, Whirtercl MC, Shams NP :
Single dose of ketotifen fumarate 0.025% VS 2 week Cromolyn
4% for allergic conjunctivitis.
28. Kidd M, Mekenzine SH, Steven J : Efficacy and safety of
ketotifen eye drops in the allergic Conjunctivitis Scientific report by
Ophthalmol, 87:1206-1211, 2003.
Correspondence:
Mohammad Reza Shoja
Shahed Beheshty Post. Po Box 583,
Yazd, IRAN.
e-mail: [email protected]
