
Multiple endpoint analysis of BAC-preserved and unpreserved
antiallergic eye drops on a 3D-reconstituted corneal epithelial
model
A. Pauly,
1,2,3
E. Brasnu,
1,2,3,4
L. Riancho,
1,2,3
F. Brignole-Baudouin,
1,2,3,5
C. Baudouin
1,2,3,4,6
(The first two authors contributed equally to this work)
1
INSERM, UMR_S968, Institut de la Vision, Paris, France;
2
UPMC University Paris 06, UMR_S 968, Institut de la Vision, Paris,
France;
3
CNRS, UMR_7210, Paris,
France;
4
Department of Ophthalmology III, Quinze-Vingts National Ophthalmology Hospital,
Paris, France;
5
Department of Toxicology, Faculty of Biological and Pharmacological Sciences, Paris, France;
6
Ambroise Paré
Hospital, APHP, University of Versailles Saint-Quentin-en-Yvelines, Versailles, France
Purpose: To compare the effects of benzalkonium chloride (BAC)-preserved and unpreserved antiallergic eye drops on
the human 3D-reconstituted corneal epithelial model (3D-HCE).
Methods: 3D-HCE were treated for 24 h followed or not by a 24 h post-incubation recovery period (24 h+24 h) with
phosphate-buffered saline (PBS), 0.01% BAC, unpreserved formulations of ketotifen, N Acetyl-Aspartyl Glutamic Acid
(NAAGA), cromoglycate, or BAC-preserved commercial formulations of ketotifen, olopatadine, epinastine, and
levocabastine. The 3D-HCE viability was evaluated using the 3-(4,5-Dimethylthiazol-2-yl) -2,5-Diphenyltetrazolium
Bromide (MTT) test at 24 h and 24 h+24 h. At 24 h, the numbers of Cluster of Differentiation 54 (CD54)- and Ki67-
immunopositive cells as well as the number of apoptotic deoxynucleotidyl transferase-mediated dUTP nick-end labeling
(TUNEL)-positive cells were evaluated on 3D-HCE frozen sections. The expression of the tight junction-associated
protein occludin was also assessed using fluorescence confocal microscopy on flat-mounted 3D-HCE epithelia.
Results: The MTT and the TUNEL tests revealed a significant decrease of cell viability and an increased apoptosis in the
superficial layers of the 3D-HCE only when treated with BAC-containing formulations and in a BAC concentration-
dependent manner. The expression of CD54 and Ki67 in the basal layers was also increased in this group. A concentration-
dependent disorganization of occludin distribution in the epithelium treated with BAC-containing solutions was also
observed. The unpreserved formulations induced effects comparable to the control.
Conclusions: BAC-preserved solutions decreased cell viability and induced apoptosis in a concentration-dependent
manner. Moreover, they induced CD54 expression, proliferation in the basal layers, and changes in the distribution of
occludin, which is consistent with a disorganization of the tight-junctions and suggests the loss of the epithelial barrier
function. On the contrary, the unpreserved solutions did not impair cell structures and viability, suggesting a better
tolerance for the ocular surface. As allergic patients often exhibit impaired and inflammatory ocular surface, BAC-free
compounds should be the first choice when treating allergic conjunctivitis.
To limit and counteract the clinical manifestations of
allergic
diseases,
antiallergic
compounds can be used. One of
these molecules, ketotifen fumarate, has demonstrated both
H1-receptor antagonism and mast cell stabilizing properties
while inhibiting chemotaxis and eosinophil activation [1,2].
Moreover, ketotifen fumarate was shown to be well tolerated
and effective in reducing the signs and symptoms of allergic
conjunctivitis [3-6]. Allergic conjunctivitis, however, has
often a tendency to become chronic, due to repeated allergic
challenge or progressive impairment of the tear film and
ocular surface [7,8].
Correspondence to: Pr Christophe Baudouin, Department of
Ophthalmology III, Quinze-Vingts
National Ophthalmology
Hospital, 28, rue de Charenton, 75012, Paris, France ; Phone:
+33.1.40.02.13.01; FAX: +33.1.40.02.13.99 ; email:
As preservatives are usually used to prevent multidose
eyedrop microbial contamination,
their chronic
administration may cause further ocular surface changes, at
the levels of tear film and conjunctiva. They can induce
cytotoxic effects and deleterious reactions when used over
long-term periods. Indeed, the mostly used preservative
benzalkonium chloride (BAC) was already shown to exhibit
toxic and inflammatory effects in clinical, in vivo and in vitro
studies [9-20]. Chronic use of BAC in eye drops is known to
be responsible for apoptosis and oxidative stress on
conjunctival cells, and to induce conjunctival inflammation
that has demonstrated potentially harmful effects on glaucoma
outcome, e.g., on glaucoma surgery efficacy [17,21-25].
In this context, the implementation of very sensitive tools
to predict eye tolerance is critical for ophthalmologists, who
may be faced with long-term induced toxicity of substances
Molecular Vision 2011; 17:745-755 <http://www.molvis.org/molvis/v17/a85>
Received 1 February 2011 | Accepted 10 March 2011 | Published 16 March 2011
© 2011 Molecular Vision
745

used at low concentration in ophthalmic preparations.
Supplied by SkinEthic
®
Laboratories (Nice, France), the
reconstructed three-dimensional (3D) model of human
corneal cells (3D-HCE) is an appropriate model for pre-
screening or investigating the undesirable effects of
ophthalmic drugs. It constitutes an interesting alternative to
animal testing that is time-consuming and often invasive and
may lack suited sensitive tools able to detect subclinical
reactions [26-28]. Multi-endpoint analyses using adapted and
improved techniques on such 3D-models have already proved
efficacy for the assessment of BAC toxicity [28] and eyedrop
tolerance [27].
The objective of this study was to investigate a large range
of commonly used antiallergic eye drops in this 3D-HCE
system and compare the tissue changes after treatment with
BAC-preserved commercial formulations of ketotifen,
olopatadine, epinastine or levocabastine, and unpreserved
commercial formulations of ketotifen, N Acetyl-Aspartyl
Glutamic Acid (NAAGA), or cromoglycate. Particularly, our
purpose was to determine the involvement of BAC in
epithelial cell damage induced after treatment with BAC-
preserved and unpreserved antiallergic eyedrops.
METHODS
Tissue model and antiallergic solution treatments: The 3D-
HCE model (SkinEthic
®
Laboratories, Nice, France) consists
of immortalized HCE cells grown vertically on a 0.5 cm
2
insert
permeable polycarbonate filter. All the experiments were
conducted as published previously [27-29]. Thirty microliters
of each solution were applied on the apical surface of 3D-
HCEs for 24 h and 24 h followed by 24 h additional recovery
time: sterile phosphate-buffered saline (PBS) used as negative
control solution, BAC solutions at 0.01% used as positive
control, the commercial solutions of 0.01% BAC-containing
ketotifen fumarate 0.025% (Zaditen
®
; Novartis Pharma SAS,
Rueil-Malmaison, France), 0.01% BAC-containing
olopatadine chlorhydrate 0.1% (Opatanol
®
; Patanol
®
; Alcon,
Ft. Worth, TX), 0.01% BAC-containing epinastine
chlorhydrate 0.05% (Purivist
®
; Allergan, Irvine, CA), 0.015%
BAC-containing levocabastine chlorhydrate 0.05%
(Levophta
®
; Chauvin Bausch & Lomb, Montpellier, France),
preservative-free ketotifen fumarate 0.025% (Zalerg
®
; Thea,
Clermont-Ferrand, France), preservative-free NAAGA 4.9%
(NAABAK
®
; Thea) and preservative-free sodium
cromoglycate 2% (Cromabak
®
; Thea; Table 1).
The recovery period (24 h) was chosen to assess the
potential reversibility of toxic effects on 3D-HCE. Six series
of 3D-HCE were used for each solution: two series for cell
viability 3-(4,5-Dimethylthiazol-2-yl) -2,5-
Diphenyltetrazolium Bromide (MTT) testing, two series for
histomorphologic analyses after hematoxylin and eosin
staining and immunohistological analyses on cryosections,
and two series for immunofluorescent labeling on the most
superficial layers of 3D-HCE by en-face confocal
microscopic analyses.
Modified MTT test: The modified MTT test was used to assess
cellular viability as described previously [27-29].
Experiments were conducted in duplicate. The 3D-HCEs were
transferred in 24-well plates containing 300 μl of the MTT
solution diluted at 0.5 mg/ml in culture medium and 300 µl of
the same MTT solution were applied on the apical surface of
the 3D-HCEs. Reconstituted tissues were incubated for 3 h.
Then, the 3D-HCEs
were transferred into 24-well plates
containing 750 µl isopropanol, and 750 µl isopropanol were
added to the apical surface of the 3D-HCEs. After a 2-h
agitation, solutions were vigorously homogenized before
reading the absorbance at 570 nm versus 690 nm. Results were
expressed as a percentage of cell viability compared to the
negative control, PBS. Analyses were performed using Safire
technology (Tecan, Lyon, France).
Confocal immunofluorescence analyses on cryosections and
entire epithelia: After incubation with the 9 different
solutions, the 3D-HCE samples were transferred into Petri
dishes containing PBS to be separated into two pieces using
a surgical scalpel. Each eceip fo tissue was e mbedded in
OCT
®
medium (Tissue-Tek, Miles Inc., Elkhart, IN), and
frozen at –80 °C. Vertical cryosections (10 μm thick) were
then cut using a cryotome (Leica CM 3050s, Leica
Microsystems AG, Wetzlar, Germany). The cryosections
were fixed in 4% paraformaldehyde (PFA) for 20 min
before immunofluorescent labeling of the tight junction
protein occludin.
Detection of apoptosis (TUNEL assay), inflammation (CD54)
and proliferation (Ki67) on 3D-HCE cryosections:
Apoptosis, TUNEL assay—Apoptosis in the tissue
layers was detected using a terminal deoxynucleotidyl
TABLE 1. BENZALKONIUM CHLORIDE (BAC) AND ACTIVE COMPOUND CONTENT OF THE ANTIALLERGIC EYE DROPS TESTED.
Eye drops
Active compound content BAC content
Ketotifen fumarate (Zaditen®; Novartis Pharma SAS,Rueil-Malmaison, France) 0.025% 0.01%
Olopatadine chlorhydrate (Opatanol®; Patanol®; Alcon, Ft. Worth, TX) 0.1% 0.01%
Epinastine chlorhydrate (Purivist®; Allergan, Irvin, CA) 0.05% 0.01%
Levocabastine chlorhydrate (Levophta®; Chauvin Bausch & Lomb, Montpellier, France) 0.05% 0.015%
Preservative-free ketotifen fumarate (Zalerg®; Thea, Clermont-Ferrand, France) 0.025% -
Preservative-free NAAGA (NAABAK®; Thea, Clermont-Ferrand, France) 4.9% -
Preservative-free sodium cromoglycate (Cromabak®; Thea, Clermont-Ferrand, France) 2% -
Molecular Vision 2011; 17:745-755 <http://www.molvis.org/molvis/v17/a85> © 2011 Molecular Vision
746
transferase-mediated dUTP-nick end labeling (TUNEL) kit
containing TUNEL enzyme and TUNEL label (Roche
Diagnostics, Meylan, France). Nuclei were stained with 4',6-
diamidino-2-phenylindole (DAPI) and the cryosections were
mounted in an anti-fade medium (Vectashield; Vector
Laboratories, Burlingame, CA).
CD54 (ICAM-1) and Ki67 immunostaining—First,
samples were fixed with 4% PFA for 10 min. Then, samples
were permeabilized with 0.01%-diluted Triton X100
®
(Sigma
Chemical Company, St. Louis, MO) for 5 min. Cells were
incubated in presence of the mouse anti-human cluster of
differentiation 54 (CD54) (IgG1; 1:100 final dilution; BD
Biosciences, PharMingen, San Diego, CA), the mouse anti-
human Ki67 (1:25 final dilution; Immunotech, Marseilles,
France) or with the isotypic control mouse IgG1 (BD
Biosciences) primary antibodies. Alexa 488 conjugated-goat
anti-mouse IgG (Invitrogen-Molecular Probes, Eugene, OR)
was used as second antibody at a 1:500 dilution. Nuclei were
labeled with propidium iodide (PI) and cryosections were
mounted in Vectashield. Samples were analyzed under a laser
confocal microscope equipped with a digital camera (E800;
PCM 2000; Nikon, Champigny-sur-Marne, France).
Immunopositive cells were then counted under the 20×
objective of the microscope in three different areas. Results
were calculated as the average of counts, and finally expressed
as cell numbers per mm of epithelial length (mm.E.L.) after
each treatment.
Confocal immunofluorescence on entire epithelia for
tight junction staining—The rabbit anti-human occludin
(IgG1; 1:100 dilution; Dako, Glostrup, Denmark) was used
for tight junction staining. Alexa 488-conjugated goat anti-
rabbit was used as second antibody. Samples were then
analyzed under a laser confocal microscope (E800; PCM
2000; Nikon) for detecting occludin expression.
Quantification and statistical analysis: Quantification of
TUNEL-, ICAM-1-, and Ki67-positive cells was performed
manually, using a microscopic grid on images under 400×
magnification. Results were expressed as mean cell numbers
per millimeter of epithelial length (mm.E.L). Standard
deviations were indicated. Statistical comparisons were
performed using two-way analysis of variance (ANOVA),
followed by multiple pairwise comparisons using the Fisher’s
adjustment (Statview V for Windows; SAS Institute, Cary,
NC).
RESULTS
Cell viability: MTT test: The PBS negative control did not
affect the cell viability neither at 24 h nor after the 24 h-
recovery period (24 h+24 h; Figure 1). The unpreserved
formulation of ketotifen fumarate KETO-BAC(-) showed the
same level of cell viability as PBS at 24 h (99,4%) and a slight
decrease of viability after 24 h+24 h (86.9% of the control).
The preservative-free formulations of NAAGA and
cromoglycate, NAA-BAC(-) and CRO-BAC(-), also showed
a weak decrease of cellular viability at 24 h (93.2% and 95.1%,
respectively) and after 24 h+24 h (87.7% for both; Figure 1).
Conversely, as expected according to previous studies
[28], 0.01% BAC showed a significant decrease of cell
viability at 24 h and after 24 h+24 h (59.6% and 55% viability,
respectively). Cell viability decreased in a BAC-
concentration dependant manner for the BAC-containing
antiallergic formulations, with a highest toxicity observed
with the 0.015% BAC-containing levocabastine chlorhydrate
0.05% [LEVO-BAC(+)]. Cell viability levels were 66.8% at
24 h and 55.3% at 24 h+24 h for 0.01% BAC-containing
ketotifen fumarate 0.025% [KETO-BAC(+)], 63.7% at 24 h
and 60.5% at 24 h+24 h for 0.01% BAC-containing
olopatadine chlorhydrate 0.1% [OPA-BAC(+)], 52.1% at 24
h and 56.6% at 24 h+24 h for 0.01% BAC-containing
epinastine chlorhydrate 0.05% [EPI-BAC(+)], and 46.3% at
24 h and 44.3% at 24 h+24 h for LEVO-BAC(+).
Immunofluorescence analyses and quantification of apoptosis
(TUNEL): Few apoptotic cells were observed after PBS
incubation (6.4 cells/mm.E.L.). Similar levels of apoptosis
were observed with the 3 unpreserved antiallergic
formulations (Figure 2): 9.2 cells/mm.E.L. for NAA-BAC(-),
12.2 cells/mm.E.L. for CRO-BAC(-), and 8.6 cells/mm.E.L.
for KETO-BAC(-), without any statistically significant
difference compared to PBS.
Consistent with previously published reports with the
same technique [28,29], BAC at 0.01% significantly increased
the number of TUNEL-positive cells compared to PBS
(p<0.0014). Apoptosis also increased on cells treated with all
BAC-containing antiallergic formulations, with a statistically
significant difference compared to PBS (p<0.0014): 28, 29.3,
46.6, and 75.5 cells/mm.E.L. for KETO-BAC(+), OPA-
BAC(+), EPI-BAC(+), and LEVO-BAC(+), respectively.
Immunofluorescence analyses and quantification of the
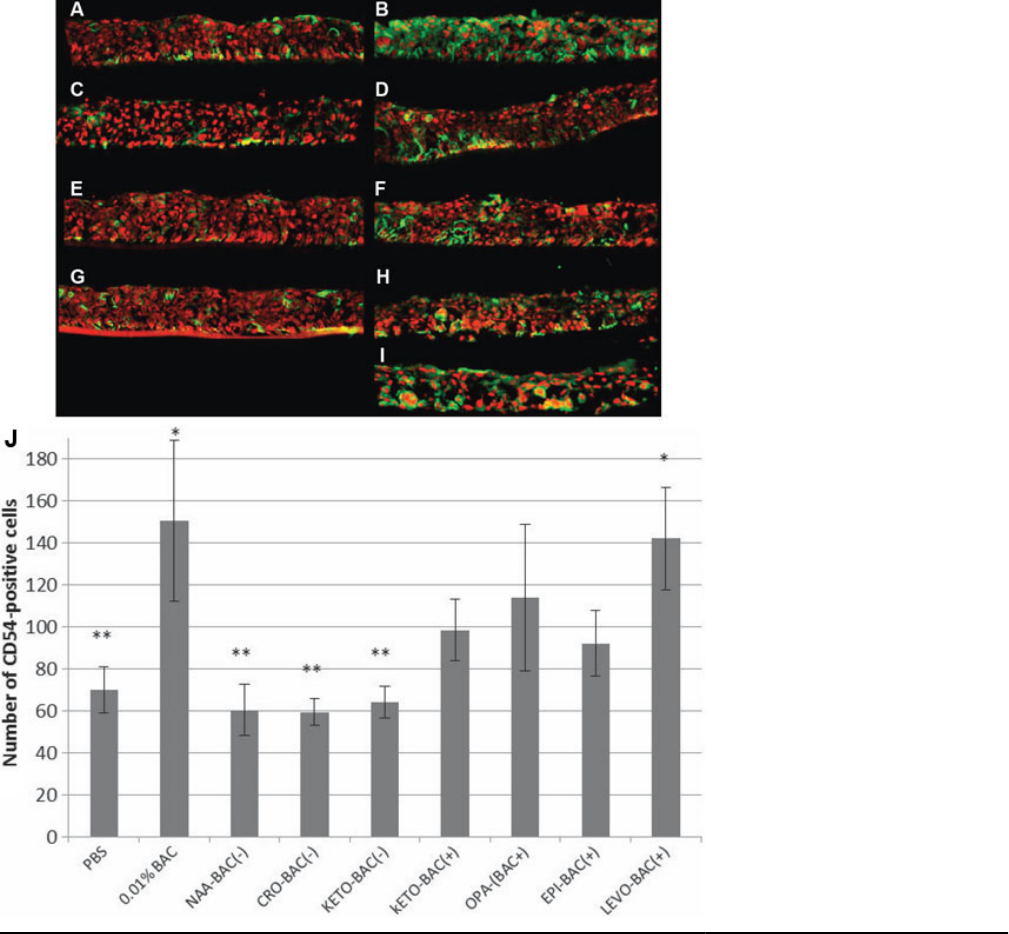
inflammation marker ICAM-1 (CD54): CD54 expression was
measured at 70 cells/mm.E.L. on PBS-treated 3D-HCE
cultures (Figure 3). The three unpreserved antiallergic
formulations NAA-BAC(-), CRO-BAC(-), and KETO-
BAC(-) expressed CD54 at low levels too, respectively, 60.3,
59.3, and 64 cells/mm.E.L., with no statistically significant
differences compared to PBS. Conversely, 0.01% BAC and
LEVO-BAC(+) showed increased levels of CD54 expression
with a statistically significant difference compared to PBS
(p<0.001): 150.4 cells/mm.E.L. for 0.01% BAC and 142 cells/
mm.E.L. for LEVO-BAC(+). KETO-BAC(+), OPA-BAC(+),
EPI-BAC(+) showed increased levels of CD54 expression
too, but no statistically significant difference was found
neither with PBS nor with 0.01% BAC i.e., 98.5, 114 and 92
cells/mm.E.L. for KETO-BAC(+), OPA-BAC(+) and EPI-
BAC(+), respectively.
Immunofluorescence analyses of cell proliferation marker
Ki67: After PBS treatment (Figure 4), few proliferating cells
were observed (29.2 cells/mm.E.L), scattered throughout the
Molecular Vision 2011; 17:745-755 <http://www.molvis.org/molvis/v17/a85> © 2011 Molecular Vision
747

entire epithelium. Similar findings were observed with the
unpreserved antiallergic treatments, with
no statistically
significant differences compared to PBS: 28.3, 30.0, and 27.3
cells/mm.E.L. for NAA-BAC(-), CRO-BAC(-), and KETO-
BAC(-), respectively (Figure 4). Conversely, numerous
proliferating cells, with a greater number located in the basal
layer, were found after 0.01% BAC, KETO-BAC(+), OPA-
BAC(+), and EPI-BAC(+) with a statistically significant
difference (p<0.04) compared to PBS: 55.3, 45, 45, and 55
cells/mm.E.L., respectively. With LEVO-BAC(+), no
proliferative cells were observed, most likely due to the deep
impairment of corneal cells as this group showed the most
important number of apoptotic cells.
En-face confocal microscopic analysis of the tight junction-
associated protein occludin: En-face confocal microscopic
analysis of 3D-HCE cultures treated with PBS, NAA-BAC(-),
CRO-BAC(-), and KETO-BAC(-) revealed a fine membrane
immunostaining of occludin in large superficial cells, forming
a ring around the cells (Figure 5). This kind of occludin
expression clearly disappeared after treatment with either
0.01% BAC, KETO-BAC(+), OPA-BAC(+), EPI-BAC(+), or
LEVO-BAC(+), all showing damaged cells with non-specific
staining.
DISCUSSION
In this study, the toxicological model of 3D-reconstructed
cornea was very helpful to demonstrate the effects of BAC-
preserved solutions on corneal cells in vitro, showing
increased
apoptosis, CD54 expression, proliferation in the
basal layers and changes in the distribution of occludin
induced with BAC-containing antiallergic treatments. On the
contrary, the unpreserved ketotifen, NAAGA and
cromoglycate solutions did not impair cell structures and
viability, suggesting a better tolerance for the ocular surface.
The highly differentiated, three-dimensional epithelial
system of human ocular origin is a desirable model for pre-
screening or investigating the effects of ophthalmic drugs. It
frees the experimenter from interspecies differences and
allows a better approach to the ocular epithelial physiology
than monolayer models and cells originating from other
organs. It also constitutes an interesting alternative to animal
testing, respecting the ethical guidelines of animal
experimentation, especially the 3R rule (refining, reducing
and replacing the use of animals) [30,31]. The reconstructed
three dimensional (3D) model of human corneal cells (3D-
HCE), supplied by SkinEthic
®
Laboratories, was found to
resemble the corneal epithelium of the human eye in
morphology and thickness [32]. Such a 3D-system models is
not only useful to demonstrate the different effects of toxic
substances on specific cell types, but also shows the
interactions between the cells and the spatial effects induced
by the toxic. Moreover, epithelium cultures at the air-liquid
interface are easy-to-handle and facilitate in vivo-like product
Figure 1. Cell viability MTT test.
Cellular viability of 3D-HCEs
treated
with PBS, 0.01% BAC, NAA-BAC(-),
CRO-BAC(-), KETO-BAC(-), KETO-
BAC(+), OPA-BAC(+), EPI-BAC(+),
or LEVO-BAC(+) for 24 h followed or
not by a 24 h post-incubation period (24
h+24 h-recovery). BAC induced a
concentration-dependent decrease of
cellular viability. At 24 h, the
unpreserved NAA-BAC(-), CRO-
BAC(-), and KETO-BAC(-)
formulations induced a slight or
insignificant decrease of cellular
viability, while the KETO-BAC
(+), OPA-BAC(+), EPI-BAC(+), and
LEVO-BAC(+) BAC-containing
formulations induced a marked decrease
of cellular viability compared to control.
After the 24-h recovery period, the
unpreserved formulations showed a
weak additional decrease of cellular
viability, while the BAC-containing
formulations still induced a strong
decrease of cellular viability compared
to control, showing irreversible damage
to 3D-HCE. Results are expressed as
percentage of cell viability compared to
the PBS control.
Molecular Vision 2011; 17:745-755 <http://www.molvis.org/molvis/v17/a85> © 2011 Molecular Vision
748

exposures. The 3D-HCEs were found to express cytokeratin-3
and include hemidesmosomes
within the basal layers.
Furthermore, they can inhibit the flow of ionic material such
as Na-fluorescein across their surface [32,33], suggesting the
presence of a functional epithelial barrier. Different types of
intercellular junctions have been identified in the corneal
epithelium ex vivo. Among them, adherens junctions,
comprising the E-cadherin protein, serve to anchor cells
together [34]. Also, the tight-junctions, originally defined as
zonula occludentes (ZO) and comprising occludin, ZO-1 and
other proteins, are thought to provide the hydrophobic barrier
preventing the free passage of molecules between adjacent
Figure 2. Apoptosis analysis (TUNEL).
Localization of TUNEL
positive cells
(green) on 3D-HCE samples after 24 h
of incubation with PBS (A), 0.01% BAC
(B), KETO-BAC(-) (C), KETO-
BAC(+) (D), NAA-BAC(-) (E), OPA-
BAC(+) (F), CRO-BAC(-) (G), EPI-
BAC(+) (H), LEVO-BAC(+) (I).
Nuclei were stained with DAPI (blue).
No or very rare apoptotic cells were
observed after PBS (A), KETO-BAC(-)
(C), KETO-BAC(+) (D) and NAA-
BAC(-) (E) treatments. KETO-BAC(+)
(D) and OPA-BAC(+) (F) induced
moderate expression of apoptosis, and
0.01% BAC (B), EPI-BAC(+) (H) and
LEVO-BAC(+) (I) induced a greater
number of TUNEL-positive cells
principally in the apical cell layers, and
also in the middle epithelial layers with
EPI-BAC(+) (H) and LEVO-BAC(+)
(I). Deeper modifications were
observed with 0.015% BAC-containing
LEVO-BAC(+) (I) compared to 0.01%
BAC (B), with a greater number of
TUNEL-positive cells in the middle
epithelial layers and a higher level of
vacuolization in the basal epithelial
layers observed with LEVO-BAC(+)
(I). The quantification of apoptotic cells
with the TUNEL assay (J) showed that
apoptotic cell number increased in a
BAC concentration-dependent manner.
BAC at 0.01% and the four BAC-
containing formulations KETO-
BAC(+), OPA-BAC(+), EPI-BAC(+)
and LEVO-BAC(+) showed much
higher expression of apoptotic TUNEL-
positive cells than did the unpreserved
formulations NAA-BAC(-), CRO-
BAC(-), KETO-BAC(-) at 24 h. Results
are expressed as cell number per mm of
epithelial length (mm.E.L.): Mean±SD
*Statistically significant compared to
PBS with p<0.0014. **Statistically
significant compared to 0.01% BAC
with p<0.0014. †Statistically significant
compared to EPI-BAC(+) with
p<0.0014. $Statistically significant
compared to LEVO-BAC(+) with
p<0.0014.
Molecular Vision 2011; 17:745-755 <http://www.molvis.org/molvis/v17/a85> © 2011 Molecular Vision
749
epithelial cells [35-37]. In a
previous study [28], we developed
a new procedure of the classical MTT test used on 3D-
reconstituted epidermal and corneal models to evaluate the
viability. This procedure showed increased sensitivity levels
and allowed detecting slight damage even in the most
superficial layers. Therefore, it is well suited to the prediction
of low to very low irritant potential, especially when products
are used repeatedly during long-term periods of time, like in
allergic conjunctivitis, when repeated allergenic challenge or
ocular surface impairment occur and require sustained
therapy.
Although the morphological relevance and sensitivity of
the 3D-HCE model allowed the modeling of cumulative
effects that may approach conditions obtained after long-term
application of eye-drops [27], our in vitro findings cannot
fully be extrapolated to in vivo conditions. Indeed, preserved
eye drops may be less toxic in vivo, according to the
continuous action of the eyelids, the permanent renewal of
ocular surface epithelia, and the presence of the preocular
mucin layer and glycocalyx. Conversely, the accumulation of
BAC-containing eye drops in the eye and the long-term use
of eye drops in allergic patients with ocular surface disorders
will emphasize the risk of toxic reactions and further
contribute to inflammatory stimulation throughout the ocular
surface, at least at a subclinical level [38].
In the present study, using our modified MTT procedure,
we evaluated the effects of either preserved or unpreserved
antiallergic formulations on cellular viability and correlated
Figure 3. Inflammation analysis.
Immunolocalization of CD54
(ICAM-1) positive cells (green) on 3D-
HCE samples after 24 h of incubation
with PBS (A), 0.01% BAC (B), KETO-
BAC(-) (C), KETO-BAC(+) (D), NAA-
BAC(-) (E), OPA-BAC(+) (F), CRO-
BAC(-) (G), EPI-BAC(+) (H), LEVO-
BAC(+) (I). Nuclei were stained with
propidium iodide (PI, red). PBS (A),
KETO-BAC(-) (C), NAA-BAC(-) (E)
and CRO-BAC(-) (G) showed a weak
expression of CD54. A significant
increase of CD54 expression was
observed after the treatments with
0.01% BAC (B) and LEVO-BAC(+)
(I), showing a green staining in all the
epithelial layers. LEVO-BAC(+) (I)
showed deeper modifications with a
higher loss of continuity between cells
and a higher level of vacuolization
observed in the basal epithelial layers.
KETO-BAC(+) (D), OPA-BAC(+) (F)
and EPI-BAC(+) (H) showed an
intermediate CD54 expression that was
localized in all epithelial layers.
Quantification of CD54-positive cells
(J) showed a higher CD54 expression
with BAC at 0.01% or the four BAC-
containing formulations KETO-
BAC(+), OPA-BAC(+), EPI-BAC(+)
and LEVO-BAC(+) than with the
unpreserved formulations NAA-
BAC(-), CRO-BAC(-), KETO-BAC(-)
at 24 h. *Statistically significant
compared to PBS with p<0.001.
**Statistically significant compared to
0.01% BAC with p<0.001. Results are
expressed as cell number per mm of
epithelial length (mm.E.L.): Mean±SD.
Molecular Vision 2011; 17:745-755 <http://www.molvis.org/molvis/v17/a85> © 2011 Molecular Vision
750

these results with those of a TUNEL assay performed on 3D-
HCE frozen sections.
Then, we investigated on entire 3D-
HCE and using en-face confocal microscopy the changes of
expression and spatial distribution of cellular markers
involved in intercellular junctions such as occludin after the
different antiallergic treatments.
Thus, with this procedure, we were able to demonstrate
concentration-dependent cytotoxic effects of BAC at 24 h, the
absence of significant cellular viability decrease following
treatment with the ketotifen, NAAGA and cromoglycate
BAC-free formulations, and a cell viability decrease similar
to that disclosed by the 0.01% BAC treatment with the BAC-
containing antiallergic formulations of ketotifen, olopatadine,
epinastine and levocabastatine. We confirmed the toxic and
proinflammatory effects of the BAC-containing solutions
using a TUNEL assay and CD54 immunostaining performed
on 3D-HCE frozen sections and found a significantly
increased number of apoptotic cells and an increased CD54
Figure 4. Proliferation analysis.
Immunolocalization of Ki67 positive
cells (green) on 3D-HCE samples after
24h of incubation with PBS (A), 0.01%
BAC (B), KETO-BAC(-) (C), KETO-
BAC(+) (D), NAA-BAC(-) (E), OPA-
BAC(+) (F), CRO-BAC(-) (G), EPI-
BAC(+) (H), LEVO-BAC(+) (I).
Nuclei were stained with propidium
iodide (PI, red). PBS (A), KETO-
BAC(-) (C), NAA-BAC(-) (E) and
CRO-BAC(-) (G) showed a weak
expression of Ki67 in all epithelial
layers. BAC at 0.01% (B), KETO-
BAC(+) (D), OPA-BAC(+) (F), and
EPI-BAC(+) (H) showed a higher Ki67
expression in all epithelial layers too.
With LEVO-BAC(+), no Ki67 positive
cells were observed, most likely due to
the deep impairment of corneal cells
with a most likely inhibition of
proliferative capabilities of 3D-HCE
submitted at this higher concentration in
BAC. Quantification of Ki67-positive
cells was concordant with these
observations (J). *Statistically
significant compared to PBS with
p<0.04. **Statistically significant
compared to 0.01% BAC with p<0.001.
$Statistically significant compared to
the other solutions tested. Results are
expressed as cell number per mm of
epithelial length (mm.E.L.): Mean±SD.
Molecular Vision 2011; 17:745-755 <http://www.molvis.org/molvis/v17/a85> © 2011 Molecular Vision
751

expression following exposure to 0.01% BAC and BAC-
containing solutions compared
to the control. Finally, we
examined the integrity of the structural and functional barrier
conferred by the tight-junctions by assessing the distribution
pattern of the occludin protein. The tight-junctions regulate
the passive movement of fluids, electrolytes, macromolecules
and cells through the paracellular pathway, thereby
contributing to the corneal defense system and to the
maintenance of the corneal homeostasis. In the mouse cornea,
the occludin distribution pattern was already described as
altered by a detergent treatment (Triton X100) using
immunohistochemistry [39]. In a previous study, Chuan et al.
[40] showed the effects of contact lens multipurpose solutions
on the corneal cells’ barrier function using fluorescein
permeability assay and immunofluorescent staining for tight
junctions proteins (ZO-1 and occludin). Recently, we also
demonstrated that occludin mRNA expression was correlated
to BAC early toxic effects [28]. Our results were consistent
with those studies, showing the disturbance of occludin tight-
junction protein distribution after BAC-containing
antiallergic treatments.
Currently, allergic conjunctivitis incidence is increasing
in developed countries. According to Manners T et al. [41],
15% of eye related consultations in general practice are due
to allergic conjunctivitis. Recommended topical treatments
for symptoms of allergic conjunctivitis include topical mast
cell stabilizers and/or topical antihistamines (H1-receptor
antagonists). Some of the new antiallergic drugs now
available may have both effects and sometimes additional
properties, such as the ability to inhibit the expression of cell
adhesion molecules (CAMs) on the cell surface or to attenuate
inflammatory mediator release [1,3-6,42-45].
Figure 5. Tight junction-associated protein occludin. Immunofluorescence analysis of occludin expressions using en-face confocal microscopy
after treatment with PBS (A), 0.01% BAC (B), NAA-BAC(-) (C) CRO-BAC(-) (D), KETO-BAC(-) (E), KETO-BAC(+) (F), OPA-BAC(+)
(G), EPI-BAC(+) (H), LEVO-BAC(+) (I). Bar=100 µm.
Molecular Vision 2011; 17:745-755 <http://www.molvis.org/molvis/v17/a85> © 2011 Molecular Vision
752
The panel of eye drops tested in the present study was
deemed to be fairly representative of the predominantly
prescribed therapeutic antiallergic molecules at the time of
these experiments in France. Indeed, among the commercial
antiallergic eye drops, one can distinguish between two types
of formulations, according to the presence of BAC as
preservative. Currently, the antihistamines olopatadine,
epinastine and levocabastine are available only as preserved
solutions whereas ketotifen is recently accessible in both
formulations. These four antihistamines constitute a group of
comparable products from the therapeutic use viewpoint and
all of them are available as preserved solutions. We deemed
it interesting to add the preservative-free ketotifen solution in
the comparison. Naaga and cromoglycate eye drops belong to
a different class of antiallergic agents, i.e., the mast cells
degranulation inhibitors. Although they were both available
as preserved and unpreserved formulations, we chose to only
test their unpreserved formulations in order not to weigh the
experiment down all the more since the preserved eye drop
forms of these two molecules are now much less used in
therapeutics than their unpreserved counterparts. Overall, our
panel choice was conducted by the actuality of the antiallergic
armamentarium that is available to the patients.
There is currently enough evidence from clinical, in vivo,
and in vitro studies that long-term use of preserved topical
drugs may induce several deleterious effects on ocular
surface, being responsible for ocular discomfort, tear film
instability, conjunctival inflammation, subconjunctival
fibrosis and epithelial apoptosis [9]. Several studies have
confirmed the participation of high concentrations of BAC-
preserved eye drops in induction of ocular surface
inflammation, allergy, fibrosis, punctate corneal staining, and
dry eye syndrome [9,38,46,47]. Three mechanisms have been
described: detergent effects inducing loss of tear film stability;
immunoallergic reactions; and direct toxic effects to epithelial
cells [48,49]. Other experimental and clinical studies have
shown that the long-term use of BAC-containing ophthalmic
solutions can induce conjunctival stroma infiltrates and
overexpression of inflammation- or apoptosis-related
molecules, such as class II antigen HLADR, ICAM-1, Fas
antigen, or the apoptotic marker Apo 2.7 [50-52].
In the present study, we showed that BAC-containing eye
antiallergic solutions may decrease cell viability, induce
apoptosis, ICAM-1 expression and proliferation in the basal
layers, and changes in the distribution of occludin.
Conversely, the unpreserved ketotifen, NAAGA and
cromoglycate formulations did not impair cell structures and
viability, suggesting a better tolerance for the ocular surface.
These findings were consistent with several previous in vitro
or ex vivo studies that demonstrated BAC toxicity and
potential advantages of BAC-free formulations [9].
Moreover, the present results support our earlier findings on
antiallergic preserved and unpreserved eye-drops. Indeed, in
a previous study, we showed that antiallergic eye drops
preserved with BAC induced high ICAM-1 expression levels,
apoptosis and oxidative stress and reduced cellular viability
in opposition to the unpreserved formulations of NAAGA and
cromoglycate [53].
In addition, our results were consistent with a recent study
by Ayaki et al. [54] on corneal and conjunctival cell lines that
showed that cell toxicity was mostly affected by the
concentration of BAC rather than the active component of
antiallergic ophthalmic solutions. The use of a large range of
commonly antiallergic eye drops in the present study was
useful to support this point, showing BAC-concentration
dependent toxic effects in all experiments. This may
emphasize the fact that epithelial toxicity was most likely
induced by the preservative (BAC) than by the active
antiallergic compound in the present study too.
These findings strongly support the use of preservative-
free solutions in patients with chronic eye diseases and
treatments over the long-term, especially in allergic
conjunctivitis or dry eye conditions. Definitely, preservative-
free antiallergic medications may decrease the adverse effects
of chronic topical medications, which could lead to better
tolerability, lower treatment discontinuations and improved
quality of life of patients with ocular allergic diseases.
ACKNOWLEDGMENTS
The study was supported by an unrestricted grant from Théa
(Clermont-Ferrand, France).
REFERENCES
1. Martín AP, Urrets-Zavalia J, Berra A, Mariani AL, Gallino N,
Gomez Demel E, Gagliardi J, Baena-Cagnani CE, Urrets-
Zavalia E, Serra HM. The effect of ketotifen on inflammatory
markers in allergic conjunctivitis: an open, uncontrolled
study. BMC Ophthalmol 2003; 3:2. [PMID: 12515585]
2. Schoch C. Effects of ketotifen 0.025% and lodoxamide 0.1%
on eosinophil infiltration into the guinea pig conjunctiva in a
model of allergic conjunctivitis. J Ocul Pharmacol Ther 2003;
19:153-9. [PMID: 12804060]
3. Borazan M, Karalezli A, Akova YA, Akman A, Kiyici H, Erbek
SS. Efficacy of olopatadine HCI 0.1%, ketotifen fumarate
0.025%, epinastine HCI 0.05%, emedastine 0.05% and
fluorometholone acetate 0.1% ophthalmic solutions for
seasonal allergic conjunctivitis: a placebo-controlled
environmental trial. Acta Ophthalmol 2009; 87:549-54.
[PMID: 18631332]
4. Avunduk AM, Tekelioglu Y, Turk A, Akyol N. Comparison of
the effects of ketotifen fumarate 0.025% and olopatadine HCl
0.1% ophthalmic solutions in seasonal allergic
conjunctivities: a 30-day, randomized, double-masked,
artificial tear substitute-controlled trial. Clin Ther 2005;
27:1392-402. [PMID: 16291412]
5. Kidd M, McKenzie SH, Steven I, Cooper C, Lanz R. Efficacy
and safety of ketotifen eye drops in the treatment of seasonal
allergic conjunctivitis. Br J Ophthalmol 2003; 87:1206-11.
[PMID: 14507747]
6. Ganz M, Koll E, Gausche J, Detjen P, Orfan N. Ketotifen
fumarate and olopatadine hydrochloride in the treatment of
Molecular Vision 2011; 17:745-755 <http://www.molvis.org/molvis/v17/a85> © 2011 Molecular Vision
753
allergic conjunctivitis: a real-world comparison of efficacy
and ocular
comfort. Adv Ther 2003; 20:79-91. [PMID:
12836808]
7. Bielory L, Friedlaender MH. Allergic conjunctivitis. Immunol
Allergy Clin North Am 2008; 28:43-58. [PMID: 18282545]
8. Wong AH, Barg SS, Leung AK. Seasonal and perennial allergic
conjunctivitis. Recent Pat Inflamm Allergy Drug Discov
2009; 3:118-27. [PMID: 19519588]
9. Baudouin C, Labbe A, Liang H, Pauly A, Brignole-Baudouin
F. Preservatives in eyedrops: the good, the bad and the ugly.
Prog Retin Eye Res 2010; 29:312-34. [PMID: 20302969]
10. Baudouin C, Liang H, Hamard P, Riancho L, Creuzot-Garcher
C, Warnet JM, Brignole-Baudouin F. The ocular surface of
glaucoma patients treated over the long term expresses
inflammatory markers related to both T-helper 1 and T-helper
2 pathways. Ophthalmology 2008; 115:109-15. [PMID:
17532048]
11. Brasnu E, Brignole-Baudouin F, Riancho L, Guenoun JM,
Warnet JM, Baudouin C. In vitro effects of preservative-free
tafluprost and preserved latanoprost, travoprost, and
bimatoprost in a conjunctival epithelial cell line. Curr Eye Res
2008; 33:303-12. [PMID: 18398704]
12. Baudouin C, Riancho L, Warnet JM, Brignole F. In vitro studies
of antiglaucomatous prostaglandin analogues: travoprost with
and without benzalkonium chloride and preserved
latanoprost. Invest Ophthalmol Vis Sci 2007; 48:4123-8.
[PMID: 17724196]
13. Liang H, Baudouin C, Pauly A, Brignole-Baudouin F.
Conjunctival and corneal reactions in rabbits following short-
and repeated exposure to preservative-free tafluprost,
commercially available latanoprost and 0.02% benzalkonium
chloride. Br J Ophthalmol 2008; 92:1275-82. [PMID:
18723745]
14. Guenoun JM, Baudouin C, Rat P, Pauly A, Warnet JM,
Brignole-Baudouin F. In vitro study of inflammatory
potential and toxicity profile of latanoprost, travoprost, and
bimatoprost in conjunctiva-derived epithelial cells. Invest
Ophthalmol Vis Sci 2005; 46:2444-50. [PMID: 15980234]
15. Ichijima H, Petroll WM, Jester JV, Cavanagh HD. Confocal
microscopic studies of living rabbit cornea treated with
benzalkonium chloride. Cornea 1992; 11:221-5. [PMID:
1587129]
16. Ishibashi T, Yokoi N, Kinoshita S. Comparison of the short-
term effects on the human corneal surface of topical timolol
maleate with and without benzalkonium chloride. J Glaucoma
2003; 12:486-90. [PMID: 14646684]
17. Buron N, Micheau O, Cathelin S, Lafontaine PO, Creuzot-
Garcher C, Solary E. Differential mechanisms of conjunctival
cell death induction by ultraviolet irradiation and
benzalkonium chloride. Invest Ophthalmol Vis Sci 2006;
47:4221-30. [PMID: 17003409]
18. Epstein SP, Ahdoot M, Marcus E, Asbell PA. Comparative
toxicity of preservatives on immortalized corneal and
conjunctival epithelial cells. J Ocul Pharmacol Ther 2009;
25:113-9. [PMID: 19284328]
19. Martone G, Frezzotti P, Tosi GM, Traversi C, Mittica V,
Malandrini A, Pichierri P, Balestrazzi A, Motolese PA,
Motolese I, Motolese E. An in vivo confocal microscopy
analysis of effects of topical antiglaucoma therapy with
preservative on corneal innervation and morphology. Am J
Ophthalmol 2009; 147:725-35. [PMID: 19181302]
20. Rolando M, Brezzo V, Giordano G, Campagna P, Burlando S,
Calabria G. The effect of different benzalkonium chloride
concentrations on human normal ocular surface. In: Van
Bijsterveld O, Lemp M, Spinelli D, editors. The Lacrimal
System. Amsterdam, Berkely, Milano: Kugler and Ghedini
Publications; 1991.
21. Baudouin C, Hamard P, Liang H, Creuzot-Garcher C,
Bensoussan L, Brignole F. Conjunctival epithelial cell
expression of interleukins and inflammatory markers in
glaucoma patients treated over the long term. Ophthalmology
2004; 111:2186-92. [PMID: 15582072]
22. Calonge M, Diebold Y, Saez V, Enriquez de Salamanca A,
Garcia-Vazquez C, Corrales RM, Herreras JM. Impression
cytology of the ocular surface: a review. Exp Eye Res 2004;
78:457-72. [PMID: 15106925]
23. Schwab IR, Linberg JV, Gioia VM, Benson WH, Chao GM.
Foreshortening of the inferior conjunctival fornix associated
with chronic glaucoma medications. Ophthalmology 1992;
99:197-202. [PMID: 1348114]
24. Broadway D, Grierson I, Hitchings R. Adverse effects of topical
antiglaucomatous medications on the conjunctiva. Br J
Ophthalmol 1993; 77:590-6. [PMID: 8218059]
25. Broadway DC, Grierson I, O'Brien C, Hitchings RA. Adverse
effects of topical antiglaucoma medication. II. The outcome
of filtration surgery. Arch Ophthalmol 1994; 112:1446-54.
[PMID: 7980134]
26. Doucet O, Lanvin M, Thillou C, Linossier C, Pupat C, Merlin
B, Zastrow L. Reconstituted human corneal epithelium: a new
alternative to the Draize eye test for the assessment of the eye
irritation potential of chemicals and cosmetic products.
Toxicol In Vitro 2006; 20:499-512. [PMID: 16243479]
27. Meloni M, Pauly A, Servi BD, Varlet BL, Baudouin C.
Occludin gene expression as an early in vitro sign for mild
eye irritation assessment. Toxicol In Vitro 2010; 24:276-85.
[PMID: 19729060]
28. Pauly A, Meloni M, Brignole-Baudouin F, Warnet JM,
Baudouin C. Multiple endpoint analysis of the 3D-
reconstituted corneal epithelium after treatment with
benzalkonium chloride: early detection of toxic damage.
Invest Ophthalmol Vis Sci 2009; 50:1644-52. [PMID:
19168896]
29. Liang H, Pauly A, Riancho L, Baudouin C, Brignole-Baudouin
F. Toxicological evaluation of preservative-containing and
preservative-free topical prostaglandin analogs on a 3D-
reconstituted corneal epithelium system. Br J Ophthalmol.
2010In press
30. Manciocco A, Chiarotti F, Vitale A, Calamandrei G, Laviola G,
Alleva E. The application of Russell and Burch 3R principle
in rodent models of neurodegenerative disease: the case of
Parkinson's disease. Neurosci Biobehav Rev 2009;
33:18-32. [PMID: 18771685]
31. ECVAM Technical Report on the Status of Alternative Methods
for Cosmetics Testing (2008–2009). A report prepared in the
framework of Directive 2003/15/EC (7th Amendment to the
Cosmetics Directive): Publications Office of the European
Union; 2010.
Molecular Vision 2011; 17:745-755 <http://www.molvis.org/molvis/v17/a85> © 2011 Molecular Vision
754
32. Nguyen DH,
Beuerman RW, De Wever B, Rosdy M.
Alternative Toxicological Methods. Salem H, Katz S, Editors:
CRC Press; 2003. p. 147–59.
33. Kahn CR, Young E, Lee IH, Rhim JS. Human corneal epithelial
primary cultures and cell lines with extended life span: in vitro
model for ocular studies. Invest Ophthalmol Vis Sci 1993;
34:3429-41. [PMID: 7693609]
34. Gumbiner BM. Cell adhesion: the molecular basis of tissue
architecture and morphogenesis. Cell 1996; 84:345-57.
[PMID: 8608588]
35. Wang Y, Chen M, Wolosin JM. ZO-1 in corneal epithelium;
stratal distribution and synthesis induction by outer cell
removal. Exp Eye Res 1993; 57:283-92. [PMID: 8224016]
36. Langbein L, Grund C, Kuhn C, Praetzel S, Kartenbeck J,
Brandner JM, Moll I, Franke WW. Tight junctions and
compositionally related junctional structures in mammalian
stratified epithelia and cell cultures derived therefrom. Eur J
Cell Biol 2002; 81:419-35. [PMID: 12234014]
37. Ban Y, Dota A, Cooper LJ, Fullwood NJ, Nakamura T, Tsuzuki
M, Mochida C, Kinoshita S. Tight junction-related protein
expression and distribution in human corneal epithelium. Exp
Eye Res 2003; 76:663-9. [PMID: 12742348]
38. Baudouin C. Allergic reaction to topical eyedrops. Curr Opin
Allergy Clin Immunol 2005; 5:459-63. [PMID: 16131924]
39. Sosnová-Netuková M, Kuchynka P, Forrester JV. The
suprabasal layer of corneal epithelial cells represents the
major barrier site to the passive movement of small molecules
and trafficking leukocytes. Br J Ophthalmol 2007;
91:372-8. [PMID: 17020902]
40. Chuang EY, Li DQ, Bian F, Zheng X, Pflugfelder SC. Effects
of contact lens multipurpose solutions on human corneal
epithelial survival and barrier function. Eye Contact Lens
2008; 34:281-6. [PMID: 18779668]
41. Manners T. Managing eye conditions in general practice. BMJ
1997; 315:816-7. [PMID: 9345194]
42. Owen CG, Shah A, Henshaw K, Smeeth L, Sheikh A. Topical
treatments for seasonal allergic conjunctivitis: systematic
review and meta-analysis of efficacy and effectiveness. Br J
Gen Pract 2004; 54:451-6. [PMID: 15186569]
43. Abelson MB. A review of olopatadine for the treatment of
ocular allergy. Expert Opin Pharmacother 2004; 5:1979-94.
[PMID: 15330735]
44. Ciprandi G, Cosentino C, Milanese M, Tosca MA. Rapid anti-
inflammatory action of azelastine eyedrops for ongoing
allergic reactions. Ann Allergy Asthma Immunol 2003;
90:434-8. [PMID: 12722967]
45. Yanni JM, Weimer LK, Sharif NA, Xu SX, Gamache DA,
Spellman JM. Inhibition of histamine-induced human
conjunctival epithelial cell responses by ocular allergy drugs.
Arch Ophthalmol 1999; 117:643-7. [PMID: 10326962]
46. Fisher AA. Allergic contact dermatitis and conjunctivitis from
benzalkonium chloride. Cutis 1987; 39:381-3. [PMID:
3581909]
47. Baudouin C, Garcher C, Haouat N, Bron A, Gastaud P.
Expression of inflammatory membrane markers by
conjunctival cells in chronically treated patients with
glaucoma. Ophthalmology 1994; 101:454-60. [PMID:
7907416]
48. De Saint Jean M, Baudouin C, Di Nolfo M, Roman S, Lozato
P, Warnet JM, Brignole F. Comparison of morphological and
functional characteristics of primary-cultured human
conjunctival epithelium and of Wong-Kilbourne derivative of
Chang conjunctival cell line. Exp Eye Res 2004; 78:257-74.
[PMID: 14729358]
49. Debbasch C, Brignole F, Pisella PJ, Warnet JM, Rat P,
Baudouin C. Quaternary ammoniums and other preservatives'
contribution in oxidative stress and apoptosis on Chang
conjunctival cells. Invest Ophthalmol Vis Sci 2001;
42:642-52. [PMID: 11222522]
50. Becquet F, Goldschild M, Moldovan MS, Ettaiche M, Gastaud
P, Baudouin C. Histopathological effects of topical
ophthalmic preservatives on rat corneoconjunctival surface.
Curr Eye Res 1998; 17:419-25. [PMID: 9561834]
51. Baudouin C, Pisella PJ, Fillacier K, Goldschild M, Becquet F,
De Saint Jean M, Bechetoille A. Ocular surface inflammatory
changes induced by topical antiglaucoma drugs: human and
animal studies. Ophthalmology 1999; 106:556-63. [PMID:
10080214]
52. Mietz H, Niesen U, Krieglstein GK. The effect of preservatives
and antiglaucomatous medication on the histopathology of
the conjunctiva. Graefes Arch Clin Exp Ophthalmol 1994;
232:561-5. [PMID: 7959096]
53. Pauly A, Brignole-Baudouin F, Guenoun JM, Riancho L, Rat
P, Warnet JM, Baudouin C. Comparative study of topical anti-
allergic eye drops on human conjunctiva-derived cells:
responses to histamine and IFN gamma and toxicological
profiles. Graefes Arch Clin Exp Ophthalmol 2007;
245:534-46. [PMID: 16900358]
54. Ayaki M, Iwasawa A, Yaguchi S, Koide R. Preserved and
unpreserved 12 anti-allergic ophthalmic solutions and ocular
surface toxicity: in vitro assessment in four cultured corneal
and conjunctival epithelial cell lines. Biocontrol Sci 2010;
15:143-8. [PMID: 21212507]
Molecular Vision 2011; 17:745-755 <http://www.molvis.org/molvis/v17/a85> © 2011 Molecular Vision
Articles are provided courtesy of Emory University and the Zhongshan Ophthalmic Center, Sun Yat-sen University, P.R. China.
The print
version of this article was created on 11 March 2011. This reflects all typographical corrections and errata to the article
through that date. Details of any changes may be found in the online version of the article.
755
