
Lee 1
Jessica Lee
AP Biology
Mrs. Kingston
23 October 2013
Abstract:
The purpose of this lab is to investigate the impact of temperature, substrate concentration,
enzyme concentration, and the presence of an inhibitor on the effectiveness and rate of an enzyme. If
the concentration of the enzyme or substrate is increased, the rate of the reaction will be increased as
well. If the temperature of the enzymes surrounding environment is increased, then the rate of the
reaction will also be increased. If the substrate concentration is increased then the rate of the reaction
will increase. If an inhibitor is added then the rate of the reaction will be decreased significantly. The
dependent variable is the rate of the reaction. The independent variable is the concentration of enzyme,
the concentration of the substrate, the temperature, and the addition of an inhibitor.
Introduction:
Enzymes are proteins that carry out chemical reactions. They are catalysts within living
organisms and regulate the rate at which a chemical reaction is carried out (Koshland). Enzymes range
in their functions throughout living organisms; however metabolic enzymes are found in all cells of the
body (Boncompagni). Enzymes are essential to the breakdown of lipids, carbohydrates, proteins and
other molecules in the cell (Koshland). There are three types of enzymes that effect the location and
function of the enzyme. These three enzymes are metabolic enzymes, digestive enzymes, and food
enzymes found in uncooked nuts, vegetables, and fruits (Boncompagni). Enzymes specifically act as a
catalyst, which is a chemical agent that is used to speed up the reaction without being used up
Lee 2
(Campbell and Recce, page 152). There are hundreds of different enzymes and the function of an
enzyme is based upon the amino acids that make up the protein. Thus enzymes present in all cells play a
major role in metabolism because without these enzymes reaction would take far too long to be carried
out. Enzymes have this ability to speed up reactions by lowering the activation energy, the energy
needed to break the bonds of the reactants (Campbell and Reece, page 152). For each reaction the
reactants must absorb energy from the surrounding to be able to break bonds. Bonds are recreated as
energy is given off to the environment. When the molecules reach the peak of the activation energy,
they are very unstable and in the transition state. When taking in energy, this normally means that the
reaction is doing so by taking in heat energy and giving off heat energy as bonds are formed again. This
means that as bonds are being broken the reaction is endothermic, meaning that it is taking in heat
energy from the surroundings, and exothermic as the bonds form, meaning that it is giving heat energy
off to the surroundings (Campbell and Reece, page 152). Exergonic is synonymous to the term
exothermic in this situation. However, in many situations there is not enough energy in the cell to
overcome this energy barrier, and if the temperature was to be increased either the proteins would be
denatured, or all the reactions would take place in the cell. Enzymes can thus speed up these reactions
and allow for them to take place (Campbell and Reece, page 153).
Enzymes are also very specific to the chemical process and will only match with
certain reactions. These reactions will take place in the active site of the enzyme. The substrate, the
reactants of the reaction, bind to the active site, the only place on the enzyme a substrate can bind,
creating an enzyme-substrate complex. The reactants are then converted to the products like in a
normal reaction, but with the enzyme, the activation energy is much lower and the rate of the reaction
is much faster. The shape of the enzymes, and active site, are a result of the amino acid sequence. As
the substrate enters the active site the chemical groups and the R groups of the enzyme interact and the
enzyme changes shape slightly to fit around the substrate even tighter. This is called induced fit
Lee 3
(Campbell and Reece, page 154). The bonds that are formed at this point are relatively weak, normally
hydrogen or ionic bonds. Enzymes have many ways in which they can lower activation energy, the first
being activating a template. The template allows the substrates to come together in the proper
orientation so that a reaction can take place (Campbell and Reece, page 154). At this point the enzyme
can stretch and bend the substrate to make it reach its transition state and break the necessary binds
for a reaction. By distorting the substrate less energy is needed to break these bonds, thus a lower
activation energy. The enzyme may also give the substrate a better environment for the specific reaction
to be carried out in that may not be present in the cell. For example, and enzyme might provide an
acidic environment over a neutral environment of a cell. Finally, the enzyme might be essential to the
reaction to take place. In some cases covalent bonds might occur between the R group and the
substrate for instances, but later the substrate will restore what was taken from the R group. Because
enzymes are not affected or changed by the substrate, enzymes can be used over and over again. The
role of the enzyme is to increase the rate of the reaction, and through these processed it does however;
other factors can also have an effect on how fast the enzyme carries out this job. For example, the more
substrate molecules that are present, the more frequently the substrate and enzyme will come in
contact. At a certain point the concentration of the substrate will fill all the active cites and by adding
more substrate the reaction is carried out unaffected (Campbell and Reece, page154). At this point the
only way to increase the rate of the reaction (when the substrate is saturated) is to add more enzymes
that the excess substrate can fill.
Temperature and pH also play a similar role in effecting the enzyme activity. As the
temperature is increased the rate of a reaction is increased to a certain point. This occurs because as
temperature is added there is more energy allowing the substrate to collide with the enzyme. Once the
temperature becomes too high the enzymes are denatured because the hydrogen bonds and ionic
bonds are disrupted. Most enzymes have an optimal temperature, and in human cells this is between
Lee 4
35-40 °C. Cells also have optimal pH values from about 6-8. Some enzymes, like those in your stomach,
function best at a much lower pH (Campbell and Reece, page 155). Along with these factors, there are
nonproteins that help the enzyme carry out this catalysis. These adjuncts are called cofactors. When
these cofactors are organic molecules they are referred to as coenzymes and vitamins are a great
example of this. While these factors most likely will enhance the performance of the enzyme there are
also enzyme inhibitors that inhibit the function of the enzymes. There are two types of inhibitors,
competitive inhibitors and noncompetitive inhibitors. Competitive inhibitors will attach to the enzyme in
the active site, which blocks the substrate from attaching to the enzyme. Noncompetitive inhibitors do
not bind to the active site of the enzyme, but rather bind to another site of the enzyme, thus changing
the shape of the enzyme and making the active site less effective in carrying out the reaction (Campbell
and Reece, page 156). These inhibitors are either reversible or irreversible. If the enzyme bonds to the
enzyme through covalent bonds, it is usually irreversible. The use of inhibitors can also be done
intentionally by the cell to regulate enzyme activity. When molecules bond to the enzyme and change
the shape of the active site this is normally allosteric regulation. This can result in either inhibition or
stimulation of an enzyme. This is seen through allosteric regulation. These enzymes are normally made
of two or more subunits with their own active sites. There are both active and inactive forms of
allosteric enzymes. If the inhibitor binds to an activator the shape is stabilized. If the inhibitor joins to an
inhibitor, it stabilizes the inactive form as well (Campbell and Reece, page 157). In another situation, the
substrate molecule that binds to the active site can stimulate catalytic powers of a multisubunit enzyme
(Campbell and Reece, page 158). When these inhibitors bind to the allosteric site, they are changing the
shape of the active site and not allowing the substrate to bind to the enzyme in many situations
(Kornberg). This can result in feedback inhibition, which occurs once so much of a certain product is
produced. This product will then switch off a metabolic pathway by this end product binding to the
enzyme that is used to receive the reactants previously in the reaction. This will bind to the allsoteric

Lee 5
site on the enzyme. This can be essential to cells because it stops the production of excess products that
just require extra expended energy to be made.
In all enzymes are extremely important to the reactions that take place within our
bodies and cells. Specific to this lab, catalase was the enzyme used and hydrogen peroxide was the
substrate used. Hydroxylamine was the inhibitor used. Catalase comes in many forms. It can protect our
red blood cells or also found in bacteria (Goodsell). Catalase is most important because of its function
and ability to break down millions of hydrogen peroxide molecules. It has four subunits, each with its
own active site (Goodsell). Hydrogen peroxide decomposes into oxygen and water in the presence of
heat or other substances. It is a colorless liquid that is commonly used for bleaching cotton and can be
corrosive to the skin if the concentration exceeds eight (Curley, Robert). Hydroxylamine is an inorganic
compound that is hygroscopic and acts as an inhibitor by binding to the enzyme, thus slowing down the
rate of the reaction significantly ("Hydroxylamine.").
Experimental Design:
This lab consisted of four different experiments that were carried out to determine the effects
of substrate concentration, enzyme concentration, and inhibitor, and temperature on enzyme activity.
For each experiment catalase from the potatoes is needed. This required the potatoes to be blended
with cold water and ice and then stored in an ice bath so that the enzyme is not denatured. For all
experiments, 1% hydrogen peroxide was needed, but there is only 6% hydrogen peroxide that was
readily available. In this case the hydrogen peroxide must be diluted. To do so a ration was needed
between the hydrogen peroxide and water. Ten mL of hydrogen peroxide must be mixed with 50 mL of
water to make a 1% dilution of hydrogen peroxide. This is used for all four trials.
In the enzyme (catalase) concentration lab collect eight beakers to prepare with eight different
concentrations of catalase (40 mL, 32mL, 30 mL, 24 mL, 20 mL, 10 mL, 4 mL, and 0mL).Add distilled

Lee 6
water to reach a total solution of 40mL. A filter paper disc is then immersed into the solution of catalase
and then placed at the bottom of each substrate solution of 1.0% hydrogen peroxide. Time how long it
takes for the disc to rise to the surface for each because this is the rate of the reaction (it rises because
of the oxygen produced from the reaction).
For the substrate concentration, prepare nine beakers of varying hydrogen peroxide
concentrations (0.0 mL, 1.3 mL, 2.7 mL, 4.0 mL, 6.7 mL, 10.7mL, 13.3 mL, 26.7 mL, 40.0 mL). Carry out
the same procedure as the enzyme concentration by putting the filter paper into a solution of 100
units/mL of enzyme then placing in the different solutions of hydrogen peroxide and measure the time it
took for the filter paper to rise to the surface.
For the enzyme inhibition lab, obtain two beakers. In one beaker put the control (40 mL of 1.0%
H
2
O
2
). In the other beaker put 5 drops of 10% hydroxylamine to 1 mL of enzyme extract. Carry out the
same procedure as before, by putting the filter paper in the inhibitor solution and then into the
substrate solution and timing how long it takes for the filter paper to rise. Repeat this twice.
For the effect of temperature lab, prepare five beakers with 40 mL of 1.0% hydrogen peroxide
and 100unit/mL enzyme concentration in another beaker. Place to 40 mL of 1.0% H
2
O
2
into a water bath
with carrying temperatures (0° C, 10° C, 22° C, 40° C, and 65° C). Use the same procedure by placing the
filter paper in the enzyme solution, then into the substrate solution of different temperatures and time
how long it takes for the filter paper to rise to the surface.
For each experiment collect the time it took for the filter paper to rise to the top and convert
this time into a rate (rate = 1/s or inverse seconds)
Results:
In this experiment there were definitive results for each section. In the enzyme concentration
lab it was determined that as the concentration of enzyme increased so did the rate of the reaction.

Lee 7
This data table can be found on page seven and the graph can be found on page nine. The graph
demonstrates the relationship between the rate of the reaction and the enzyme concentration and
there is a direct relationship between rate and enzyme concentration. In the concentration of substrate
lab, as the concentration of the hydrogen peroxide increased, so did the rate of the reaction. The data
table that demonstrates this conclusion can be found on page eight and the corresponding graph can be
found on page nine. The graph shows the direct relationship between substrate concentration and the
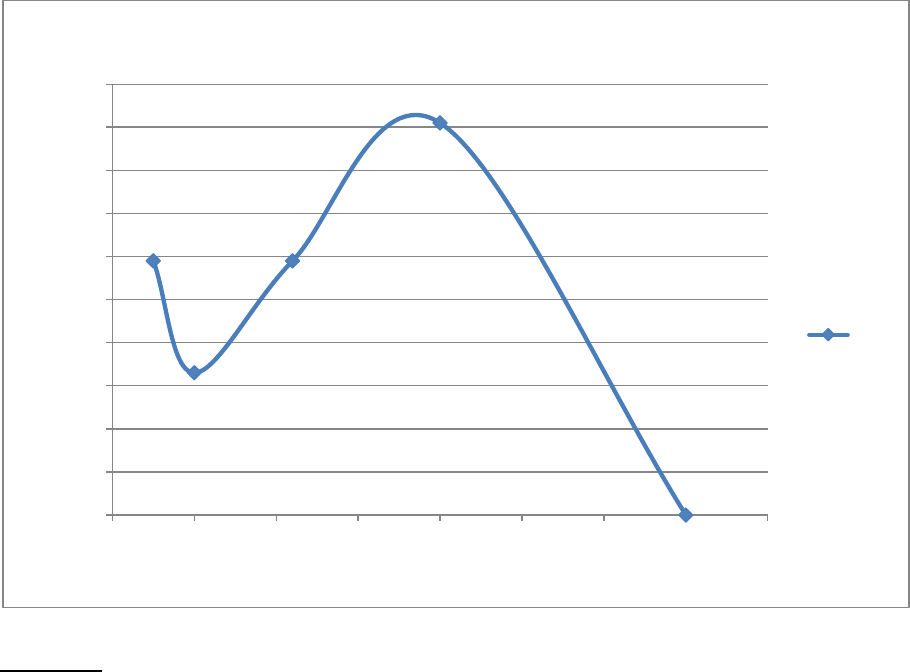
rate of the reaction. In the in temperature portion of the experiment it was determined that the rate
relatively increased as the temperature increased until the enzyme was denatured. This data table can
be found on page eight and the graph that represents this data in found on page ten. The graph shows
the direct relationship between temperature and rate of the reaction. The data for the inhibition lab can
be found on page eight and there is no graph. The data clearly presents the effect of an inhibitor and
how it significantly slowed the rate of the reaction. All data tables and graphs are found on pages seven
through ten and allow for further conclusions to be drawn.
Data:
Table 1
Effect of Enzyme Concentration of Rate of Activity
Enzyme Concentration
(units/mL)
Trial 1
(Seconds)
Trial 2
(Seconds)
Average
(Seconds)
Rate
(1/seconds)
100
2.5
3
2.75
.8
80
1
3
2
.5
75
3
4
3.5
.286
60
4.5
4.7
4.6
.217
50
4
6
5
.2
25
6
8
7
.143
10
6
7
6.5
.154
0
52
72
52
.161

Lee 8
Table 2
Effect of Substrate Concentration on Enzyme Activity
H
2
O
2
%
(Substrate)
Group 1 Time
(s)
Group 2 Time
(s)
Group 3 Time
(s)
Average Time
(s)
Rate (s
-1
)
0
N/A
N/A
N/A
N/A
N/A
0.1
16.295
20.45
38.9
25.215
0.0397
0.2
18.19
12.85
15.45
15.663
0.0638
0.3
10.31
9.45
13.4
11.053
0.0905
0.5
7.97
8.05
9.2
8.407
0.1189
0.8
6.455
6.85
7.9
7.068
0.1415
1.0
5.5
3.85
6.55
5.3
0.1887
2.0
3.02
3.8
5.3
4.04
0.2475
3.0
1.96
1.65
2.9
2.17
0.4608
Table 3
Effect of an Inhibitor on Enzyme Activity
Enzyme
Concentration
(units/mL)
Time to float disk (seconds)
(1/seconds)
Trial 1
Trial 2
Average
Rate
Control
2.5
2.3
2.4
0.417
Hydroxylamine
73.8
66
69.9
0.014
Table 4
Effect of Temperature on Rate of Enzyme Activity
Time to Float Disc (in seconds)
Temperature (Degrees C)
Trial 1
Trial 2
Average
Rate
(1/seconds)
5
9
8
8.5
0.118
10
19
11
15
0.066
22
11
6
8.5
0.118
40
7
4
5.5
0.182
70
n/a
n/a
n/a
n/a

Lee 9
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
0 20 40 60 80 100 120
Rate of Reaction (s
-1
)
Enzyme Concentration
Effect of Enzyme Concentration on Rate of
Reaction
Rate
0
0.05
0.1
0.15
0.2
0.25
0.3
0.35
0.4
0.45
0.5
0 0.5 1 1.5 2 2.5 3 3.5
Rate of Reaction (s
-1
)
Percent Hydrogen Peroxide
Effect of Percent of Hydrogen Peroxide on Rate
of Reaction
Rate
Lee 10
Discussion:
Once all this data was calculated and, investigated, and analyzed, it was evident the objectives
and purposes of the lab were attained. This objective was to determine the effects of substrate
concentration, enzyme concentration, temperature, and an inhibitor on the rate of a reaction. The final
conclusions made were that as enzyme concentration, substrate concentration, and temperature were
increased, the rate of the reaction also increased. When the inhibitor was added the rate of the reaction
decreased significantly. All four of these parts of the experiment used the reaction between catalase
and hydrogen peroxide. This reaction can be modeled as
2H
2
O
2
+ catalase 2H
2
O
+ oxygen + catalase
Catalase clearly accelerates the process of the breakdown of hydrogen peroxide into water and oxygen.
As discussed in the introduction, the enzyme catalase also remains after the reaction occurs because the
0
0.02
0.04
0.06
0.08
0.1
0.12
0.14
0.16
0.18
0.2
0 10 20 30 40 50 60 70 80
Rate of reaction (s
-1
)
Temperature (°C)
Effect of Temperature on Rate of Reaction
Rate
Lee 11
reactants do not make covalent bonds with the enzyme at the active site. This reaction is so important
to the cell because it makes sure that there is not a buildup of excess hydrogen peroxide that can disrupt
the function of cells. For each of the four sections of the lab this reaction must be taken into
consideration in order to fully understand why the results were the way they were.
The first independent variable tested was the concentration of the enzyme. Eight beakers were
used to hold different concentrations of enzymes that the filter paper was put into. Then the filter paper
was put into the substrate solution. The substrate in this case was the 1.0% hydrogen peroxide. The disc
rose much quicker as the concentration of the enzyme increased, thus the rate of the reaction was much
quicker. This can be concluded because as the reaction takes place oxygen is produced as a product.
The filter paper absorbed the oxygen and rose once this reaction took place. As a result of the higher
concentration of enzymes, the substrates had more possible active sites to bind to, thus more reactions
were able to take place at once. As the concentration increased there were more and more
opportunities for the hydrogen peroxide to bind the active sites of the catalase and react with a lower
activation energy. In the second section of the lab the concentration of the substrate was varied while
the concentration of the enzyme was kept constant. Nine beakers were prepared with differing
concentrations of hydrogen peroxide as explained in the experimental design. The filter paper was
placed in the enzyme solution and then placed into the substrate solutions and timed for how long it
took for the filter paper discs to rise to the surface. As the concentration of the substrate was increased,
the rate of the reaction also increased for reasons similar to the concentration of the enzyme. As the
concentration of substrate increased there were more substrate molecules to bond with the active site
of the catalase enzyme. Because there was this increase in the substrate concentration each time, there
was a greater likelihood that the substrate would bind with the active site and carry out the reaction.
The third section of the lab required five beakers that varied in temperature of the water bath that the
reaction took place in. The enzyme and substrate concentration were all kept constant and the filter
Lee 12
paper was placed in the enzyme and then the substrate within these different temperature
environments. As the temperature increased the rate of the reaction relatively increased as well. The
word relatively is used because the rate did decrease at first and then significantly increase as the
temperature increased. Then at 70 degrees Celsius the data in the table on pageeight displayed that
there was no applicable data because first the beaker broke because of such a high temperature, and
second the enzymes were denatured because of such high temperatures. The rate of the reaction
increased as the temperature increased because the molecules have more kinetic energy and thus the
substrate and active sites of the enzymes were colliding much more often. This means that there are
more interactions between the substrate and active sties of the enzymes as the temperature of the
water bath is increased because there are more collisions between the two. Because more reactions are
taking place more oxygen is produced and at a much faster rate, resulting in the quicker rise of the filter
paper in the hydrogen peroxide substrate solution. The final section of the lab required the investigation
of how an inhibitor, hydroxylamine, effected the rate of the reaction. Five drops of hydroxylamine were
added to the enzyme extract (catalase). This reaction was accompanied by a controlled group. It was
evident that the rate of the reaction were drastically different as a result of the inhibitor,
hydroxylamine. The rate of the reaction with this inhibitor was significantly slower than the rate of the
control group that consisted of 100untis/mL enzyme and 1.0% hydrogen peroxide. This difference in
rate is a direct result of the inhibitor. Inhibitors are used to bind to the enzyme as discussed in detail in
the introduction. Hydroxylamine bound to either the active site or and allosteric site to alter the shape
of the enzyme and the active sight of the enzyme. This means that the reaction was not able to be
catalyzed completely and the reaction was not able to continue at as fast a rate as without an inhibitor.
The active sight became less effective in catalyzing the reaction to the products of water and oxygen.
Thus less oxygen was produced because in the case of a competitive inhibitor it took the place in the
active site, or the reaction was much slower meaning that it took a significant longer time for the oxygen

Lee 13
to be produced and make the disc rise. This corresponds directly with the how inhibitors work as
explained earlier in the lab.
When looking at the graphs and data on pages seven through ten it was evident that there was
room for error in many respects. First off the, the most apparent error seemed to be in the temperature
lab. As the temperature was increased the rate of the reaction should have directly increased as well.
This was not completely apparent, because as the temper rose from five degrees Celsius to ten degrees
Celsius the rate of the reaction decreased from 0.118 (1/s) to 0.066 (1/s). This could be a result of many
errors or the fact that potatoes do not normally grow in high temperatures. This is an apparent
discrepancy because it would be expected for the rate to increase as the temperature increased. The
second place for errors could be basic human errors, while diluting the hydrogen peroxide from 6% to
1%. However, all the results followed a trend that was expected and agreed with the hypothesis that
was made. Due to this fact, none of the errors could have been that significant to completely skew the
meaning and significance of the analysis of the data. From this we can conclude that the results are
relatively valid.
Conclusion:
Overall this lab was relatively successful in determining the effects of enzyme concentration,
substrate concentration, temperature, and an inhibitor on the rate of the reaction. The objective of the
lab was met, which was the rate of the reaction for all four sections of the lab. In doing so the time for
the disc to rise to the surface was measured and then calculated into a rate. All this data that was
collected was used to agree with not only the objectives of the experiment but also the hypotheses
made on each individual lab. The results were relatively valid and showed a correct trend in data. To
make these results even more valid, it would be necessary to complete the experiment multiple more
Lee 14
times because some sections did not have multiple trial to verify the results completely. All in all the
results were reliable and showed the basic properties of enzymes and their role in chemical reactions.

Lee 15
References:
Boncmpagni, Tatiana. "Enzymes Try to Grab the Spotlight." New York Times. New York Times, 22 Feb.
2012. Web. 22 Oct. 2013.
Campbell, Niel A., and Jane B. Reece. AP Edition Biology. 8
th
ed. New York: Benjamin/Cummings, 2008.
Print.
Curley, Robert. "Hydrogen Peroxide." Encyclopedia Britanica. EnccopediaBritanica Inc., 12 Apr. 2007.
Web. 22 Oct. 2013.
Goodsell, David. "Catalase." PDB Protien Data Bank. RCB PBD Protien Data Bank, 2004. Web. 22 Oct.
2013.
"Hydroxylamine." ChEBI: The Database and Ontology of Chemical Entities of Biological Interest. Ed.
ChEBI Team. ChEBI, 21 Aug. 2013. Web. 22 Oct. 2013.
Kornberg, Hans. "Metabolism." Encyclopedia Britanica. Encyclopedia Britanica Inc., n.d. Web. 22 Oct.
2013.
Koshland, Daniel E., Jr. "Role of Enzymes in Metabolism." Encyclopedia Britanica. Ecyclopedia Britanica
Inc., n.d. Web. 22 Oct. 2013.
