
This document forms part of the ‘Evaluating environmental enrichment’ resource, developed by the NC3Rs, IAT and RSPCA to
support animal technicians to undertake robust evaluations of enrichment items and assess their impact on animal welfare. For
more information, visit www.nc3rs.org.uk/evaluating-environmental-enrichment.
1
Protocol C: Video recording zebrafish behaviour
In this document
Introduction
About the protocol
Protocol steps
Looking at the data
Introduction
The provision of enrichment for zebrafish is not as common
as it is for terrestrial research animals, with the most common
form of zebrafish enrichment being
live food, such as rotifer.
Reasons for this include issues with the practicality of
including structural enrichment within tanks and concerns
about contamination of tank (and system) water by
enrichment items.
There is an evidence base suggesting that the provision of physical enrichment can reduce certain negative
welfare indicators in zebrafish (e.g. a reduction in the ‘stress hormone’ cortisol; see Stevens et al. 2021
for a
review of the literature). However, it is perhaps more difficult for us to see the benefits of enrichment in this
species and to recognise when zebrafish are experiencing a positive welfare state, compared to mammalian
species commonly used in research.
All of these reasons make it especially important to properly evaluate environmental enrichment for zebrafish
and, if possible, to share your findings
to advance our understanding of this area.
About the protocol
This example protocol is a step-by-step guide on how you might prepare for and carry out basic behavioural
observations, using video recording equipment, to answer the questions:
Is an enrichment item used by fish?
Does the presence of enrichment affect the behaviour of the fish?

This document forms part of the ‘Evaluating environmental enrichment’ resource, developed by the NC3Rs, IAT and RSPCA to
support animal technicians to undertake robust evaluations of enrichment items and assess their impact on animal welfare. For
more information, visit www.nc3rs.org.uk/evaluating-environmental-enrichment.
2
More complex evaluations of enrichment can be found in the published literature.
Within the protocol
*Rand
indicates an opportunity to incorporate >randomisation< into your study, and
*Flex
indicates a part of the protocol that is flexible depending on your circumstances (e.g. how many animals/tanks
are available to study).
General advice is given on how to look at the data generated by this protocol in order to draw conclusions
about the questions of interest. More detailed instructions on using MS Excel to look at data and insert charts
can be found in Protocols A, B and D. If you would like to carry out statistical tests on your data, we
recommend consulting someone with statistical expertise before you begin data collection.
More information on behaviour observations
can be found in the ‘Evaluating environmental enrichment’
resource. Published studies that include behaviour observations can be found on the ‘Example enrichment
study protocols’ page.
Protocol steps
1. Define your behaviours of interest
2. Select the tanks to observe
3. Assign the tanks to a treatment
4. Create a schedule of observations
5. Add the enrichment to the tanks
6. Allow the fish to acclimatise to the enrichment
7. Have a dry run with your equipment
8. Make your recordings
9. Back up your recordings
10. Watch the recordings back
11. Input your data onto a spreadsheet
1. Define your behaviours of interest
In this example two measures will be used to evaluate the enrichment:
The number of interactions with the enrichment items.
The number of aggressive interactions observed.
More measures can be added but remember that the number and type of behaviours of interest will need to
be manageable for you to observe and record.
As you will be video recording behaviour, it will be possible to re-watch the videos if you decide to record the
occurrence of another behaviour at a later date.

This document forms part of the ‘Evaluating environmental enrichment’ resource, developed by the NC3Rs, IAT and RSPCA to
support animal technicians to undertake robust evaluations of enrichment items and assess their impact on animal welfare. For
more information, visit www.nc3rs.org.uk/evaluating-environmental-enrichment.
3
In order to collect data consistently, the behaviours must be well defined; similar to when creating an
ethogram. Define which behaviours will be counted as aggressive interactions and which will not. The
examples shown in Table 1.1 were created using the general zebrafish ethogram.
Behaviour of interest
Definition
Interact with enrichment
Passing through the enrichment item, making contact with it, or resting
underneath it.
Aggressive interactions
One fish moves towards a second fish, increasing acceleration, while the
second fish avoids the first. Mouth opening and biting may occur. Erratic
swimming patterns may be observed, such as the pursued fish making sharp
(as opposed to smooth) turns in an attempt to flee from the aggressor.
Do not count non-aggressive following behaviour, i.e. no increased
acceleration, no lunging or striking towards others observed. The other fish
does not appear agitated by the follower and does not seek to escape from the
follower by swimming erratically; turns are smooth.
Do not count nose-tail touching. This is a non-aggressive social interaction,
commonly seen during courtship. A fish touches the side or tail of another fish
with the nose or head.
Table 1.1. Examples of how to clearly define which behaviours you will and will not record.
2. Select the tanks to observe
Select *
Rand
eight *
Flex
tanks to observe. Four tanks will have enrichment, and four will act as controls to gauge
the level of aggression in the absence of enrichment.
3. Assign the tanks to a treatment
Assign *
Rand
the tanks you have selected to the enriched or control category. For example, write down the
tank numbers on different scraps of paper, shuffle them and then pick them out of a container. The first four
you select can be assigned to the ‘test tank’ treatment, which will contain the new enrichment. The remaining
four will be control tanks.
4. Create a schedule of observations
Factor in time for fish to acclimatise to the presence of the new enrichment (one to two weeks), and for fish to
settle after setting up the recording equipment. Keep these periods the same for all tanks.
You may wish to look at behaviour during feeding time, or you may wish to avoid this period of excitement. In
any case, it is important to be aware of factors that may cause a disturbance and to record this information for
reference when you are interpreting your findings.
For the purpose of this example, there is only access to one camera, so recordings cannot take place
simultaneously. If it is possible to carry out simultaneous recordings, prioritise recording from a test and
control tank at the same time.
This document forms part of the ‘Evaluating environmental enrichment’ resource, developed by the NC3Rs, IAT and RSPCA to
support animal technicians to undertake robust evaluations of enrichment items and assess their impact on animal welfare. For
more information, visit www.nc3rs.org.uk/evaluating-environmental-enrichment.
4
Your schedule will depend on how many tanks you have chosen to observe and your other commitments.
You may wish to make multiple recordings in one day or spread them out over a longer period.
Table 1.2 shows a recording schedule for eight tanks (with a single camera) spread over two weeks.
Week 1
Tank
Recording slot
Test 3
Tues 10 AM
Test 2
Tues 3 PM
Control 1
Weds 10 AM
Control 2
Weds 3 PM
Week 2
Tank
Recording slot
Control 4
Mon 11 AM
Test 1
Mon 4 PM
Test 4
Tue 11 AM
Control 3
Tue 4 PM
Table 1.2. Schedule for recording fish behaviour.
Note that two control tanks and two test tanks have been assigned to the recording slots, but otherwise the
allocation was random. The observations will be spread over the day, to attempt to account for other factors
that may affect behaviour (e.g. differences in activity levels between the morning and afternoon).
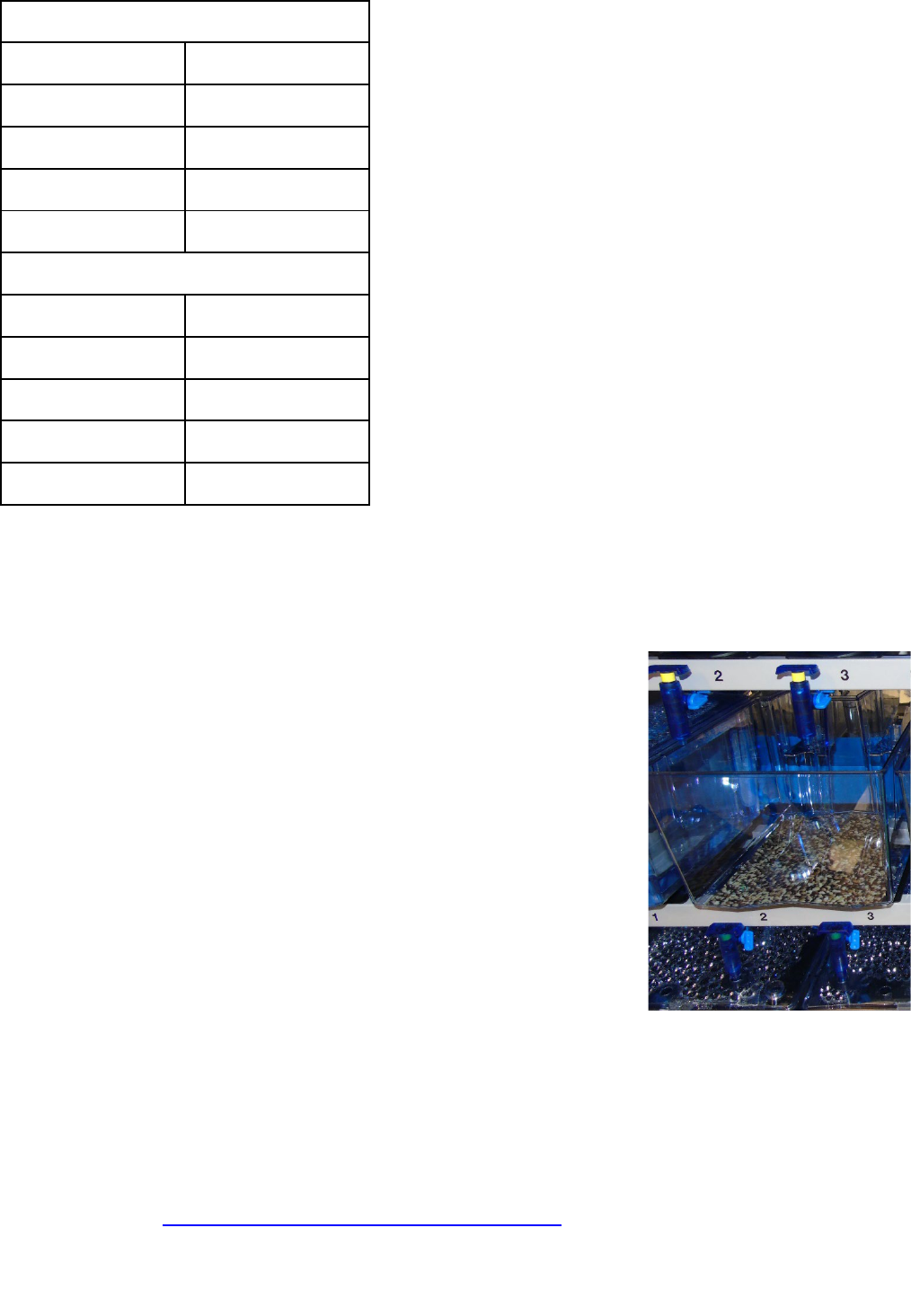
5. Add the enrichment to the tanks
The control tanks should be your current, standard tank set up, before the
enrichment you are evaluating is introduced. For this example, the control
tanks are barren, with an image of gravel placed underneath them.
To create the test tanks, add any standard items included in the control
tanks (in this case, the image of gravel) and then add the enrichment
item(s) you want to trial. For the purpose of this example, physical
enrichment (shelters) will be trialled. Note the date that the enrichment was
added to the tanks, this may be useful information (e.g. if you decide to
observe longer term responses to enrichment over time).
Two shelters will be added to each test tank in an attempt to provide
sufficient physical enrichment for the number of fish within the tank, and in
anticipation of competition over the resource.
Image credit:
Dr Will Norton,
University of Leicester

This document forms part of the ‘Evaluating environmental enrichment’ resource, developed by the NC3Rs, IAT and RSPCA to
support animal technicians to undertake robust evaluations of enrichment items and assess their impact on animal welfare. For
more information, visit www.nc3rs.org.uk/evaluating-environmental-enrichment.
5
Different combinations of enrichment items can have different outcomes, so make sure you have made a note
of exactly what each tank set up contains (e.g. control: gravel sticker only and test: gravel sticker with two
identical shelters) so that you have this information when you come to report your results.
6. Allow the fish to acclimatise to the enrichment
Studies where fish have indicated a preference for enrichment typically allow an acclimation period of several
days (Stevens et al. 2001
).
This suggests that for an accurate evaluation of enrichment items the fish will require time to settle before you
begin your observations.
Allow the fish one to two weeks between introducing the enrichment and recording data. During this period,
monitor the test tanks closely to ensure that no harm comes to the animals.
7. Have a dry run with your equipment
You will need to find the best position for the camera so that the whole tank and all the fish are visible on the
recording. This may be filming through the front of the tank or an aerial view.
Make a recording and check the video. Ensure that there is enough device memory to record for the intended
period.
8. Make your recordings
Following your schedule, carefully set up the recording equipment and begin the recording.
Leave the camera filming for one hour *
Flex
.
9. Back up your recordings
Create a backup (or two!) of your experimental videos as soon as possible (e.g. save them onto a PC and
upload them onto Cloud storage). If your device has limited space to store recordings, delete them once they
are backed up. This will prevent your next recording getting cut short due to lack of device memory.
10. Watch the recordings back
Discount the first 30 *
Flex
minutes of the video as an acclimatisation period, to account for the disturbance of
setting up the camera. Keep this period the same for all the videos.
Observe behaviour starting just after 30 minutes *
Flex
, for a period of ten minutes. Record all incidents of any
fish within the group performing
the behaviours that you previously defined.
Pause the video when you notice a behaviour of interest and record it on a data collection sheet or straight
onto an Excel spreadsheet. You may need to rewind the video to note all the behaviours, especially if more
than one fish is near to or interacting with the enrichment at once. You may want to count the interactions with
enrichment first and then play the same section of the video back again to record incidents of aggression.
Because interactions with the enrichment should be obvious, increasing the playback speed of the videos
may be possible to collect the data more quickly. If you have more than one enrichment item, it may help to
watch the video with a colleague, with each of you focusing on a different item.
Recording incidents of aggression within a group may be more challenging. If you enlist the help of
colleagues, ensure that they are clear on what should be recorded as aggression.

This document forms part of the ‘Evaluating environmental enrichment’ resource, developed by the NC3Rs, IAT and RSPCA to
support animal technicians to undertake robust evaluations of enrichment items and assess their impact on animal welfare. For
more information, visit www.nc3rs.org.uk/evaluating-environmental-enrichment.
6
Your notes might look something like Figure 1.1.
Figure 1.1. An example of a completed data collection sheet.
Note that this stage of data collection is at risk of bias
due to preconceptions about how enrichment may
affect aggression.
To avoid this, enlist the help of colleagues. Ideally these colleagues should not be aware of the purpose of the
study, but this may not be possible.
Multiple people can watch and score the same videos to help maintain observer consistency
.
11. Input your data onto a spreadsheet
At this stage it will be possible to blind
the data to minimise the risk of bias during data analysis.
Looking at the data
General advice is given how to look at the data generated by this protocol to answer the draw conclusions
about the questions of interest. If you would like to carry out statistical tests on your data, we recommend
consulting someone with statistical expertise before you begin data collection.
Remind yourself of the aims of the study. The questions of interest
are shown below with suggestions on how to approach them.
Is an enrichment item used by fish?
Following Protocol C will have generated count data on how
frequently the fish interact with the enrichment.
This will give you a general idea of whether the fish find the
enrichment useful, but it will be up to you to interpret if the number of interactions observed indicates interest.
Detailed guidance on using the
COUNTIF and SUM functions is
included in ‘Looking at your data’
for Protocol A and Protocol D.
More information on using
different types of charts can be
found in the ‘Looking at your data’
section of Protocol B.

This document forms part of the ‘Evaluating environmental enrichment’ resource, developed by the NC3Rs, IAT and RSPCA to
support animal technicians to undertake robust evaluations of enrichment items and assess their impact on animal welfare. For
more information, visit www.nc3rs.org.uk/evaluating-environmental-enrichment.
7
Collecting this data at different time points will allow you to investigate whether use of the enrichment
increases, decreases or generally stays the same at different times of the day, or over longer periods (e.g. by
comparing the number of interactions with the enrichment counted on week one vs. week six).
A change in enrichment use over time can be visualised using a figure such as a line graph.
Does the presence of enrichment affect the behaviour of the fish?
Following Protocol C will have generated count data on how frequently aggressive interactions occurred.
Find the total number of aggressive interactions observed for the Control tanks and the Test tanks and
compare these numbers.
You can visualise this data as a bar chart, or a scatterplot (with individual tanks plotted as individual data
points).
General advice on summarising your data
Use the =SUM function in Excel to give you the total number of interactions.
Use the =MEAN function in Excel to give you the mean (average) number of interactions.
SORT and FILTER the data to break down your data summary by tank.
Use Insert Charts in Excel to visualise your data. Tutorials are available online (e.g. on YouTube) if you
require extra help with this.
The basic steps for creating a chart in Excel are:
Arrange the data you want to visualise into a table.
Highlight the table using the cursor.
Select the ‘Insert’ menu.
Choose the chart you wish to plot.
Amend the chart as necessary (e.g. adding axis labels, changing the formatting or changing how the
data is presented).
What next?
Carefully consider what you have found and whether any further investigation would be useful.
Refer to the Evaluating Enrichment Resource for advice on implementing and sharing your findings
.
