SAP general 1 12/05/2011
SAMPLING AND ANALYSIS PLAN
GUIDANCE AND TEMPLATE
VERSION 4, General Projects
R9QA/009.1
May 2014
This Sampling and Analysis Plan (SAP) guidance and template is intended to assist
organizations in documenting the procedural and analytical requirements for one-time, or time-
limited, projects involving the collection of water, soil, sediment, or other samples taken to
characterize areas of potential environmental contamination. It combines the basic elements of a
Quality Assurance Project Plan (QAPP) and a Field Sampling Plan (FSP). It will meet the
requirements for any U.S. Environmental Protection Agency (EPA) Region 9 funded projects
which involve taking environmental measurements.
The format is designed for use in projects generating a limited number of samples collected over
a relatively short period of time. This template is not appropriate for on-going monitoring
events, for remediation, or removal activities. Exceptions to all of these requirements will be
considered on a case-by-case basis, but they should be discussed with the Region 9 QA Office
before the SAP is submitted for approval. This template can be used by state, municipal and
local agencies, contractor, non-profit organizations, and by EPA staff.
This guidance provides item-by-item instructions for each section. If appropriate, the language
from any section may be used as written, or modified to reflect project- and sampling-specific
requirements. Not all sections will apply to all organizations or to all projects.
Some sections, such as those describing sampling procedures, contain example language which
can be used with or without modification. If these procedures do not meet project needs, they
should be replaced by a description of the organization’s sampling procedures. If available,
copies of standard operation procedures (SOPs) for sampling may be included with the SAP.
Other alternatives should be discussed with QA Office staff.

SAP general 2 12/05/2011
An electronic version of the template is available and can be used to prepare the SAP. The
format of the template is as follows:
All instructions and tutorial information, shown in shaded italic type, should be deleted from the
final SAP.
Suggested text, which may be included as written in the SAP, is presented in normal type. This
text can be used, modified, or deleted depending on the nature of the project. For example, if the
project only involves groundwater sampling, the discussion of sampling other matrices should be
deleted. If there is more than one option, choose the appropriate one and delete the others.
Applicable SOPs can be included as an appendix to the final SAP and referenced in the
appropriate section(s) of the SAP.
An underlined blank area (___________) in the template indicates that text should be added.
Examples or choices may be in [brackets] and italic type following the blank space. If
appropriate, select one and delete the others. Use as much space as necessary to completely
address each section. Remove the underlining from the added text.
If a given section does not apply, it is recommended that it be kept in the SAP with the statement
“Not applicable” or “Does not apply” under the section heading. This avoids the writer having
to renumber sections. If sections that are not relevant to the project are removed altogether, the
remaining sections will need to be renumbered.
Example forms are provided in Attachment 1. These forms should be deleted from the final
SAP.
Please call the U.S. EPA Region 9 Quality Assurance Office for assistance in completing the
SAP. Contact Dr. Eugenia McNaughton at 415-972-3411, or Ms. Gail Morison at 415-972-
3807.

SAP general 3 12/05/2011
Sampling and Analysis Plan for
[Title of Project]____________________________________________
[Name and Address of Organization Here]_______________________
_____________
Date
[Name of Organization] Project Manager ________________________
[Name of Organization] QA Manager ________________________
For EPA use:
Approved by EPA Project Manager:
_________________________
Date:
_________________________
Expedited Review?
G
Yes
G
No
Received by QA Office:
__________________________
Date:
_________________________
Reviewed by:
__________________________
Date:
_________________________
Approved:
__________________________
Region 9 Quality Assurance Manager
Date:
_________________________
SAP general 4 12/05/2011
TABLE OF CONTENTS
1.0 INTRODUCTION ............................................... 7
1.1 Site Name or Sampling Area ................................... 7
1.2 Site or Sampling Area Location................................. 7
1.3 Responsible Agency ......................................... 7
1.4 Project Organization ........................................ 7
2.0 BACKGROUND ................................................ 9
2.1 Site or Sampling Area Description ............................... 9
2.2 Operational History ......................................... 9
2.3 Previous Investigations/Regulatory Involvement .................... 10
2.4 Geological Information ..................................... 10
2.5 Environmental and/or Human Impact ........................... 10
3.0 PROJECT DATA QUALITY OBJECTIVES ........................... 12
3.1 Project Objectives and Problem Definition ....................... 12
3.2 Data Quality Objectives (DQOs) .............................. 12
3.3 Data Quality Indicators (DQIs) and Measurement Quality Objectives (MQOs)
......................................................... 13
3.4 Data Review and Validation .................................. 15
3.5 Data Management ........................................ 16
3.6 Assessment Oversight ...................................... 16
4.0 SAMPLING RATIONALE ....................................... 19
4.1 Soil Sampling ............................................ 19
4.2 Sediment Sampling ........................................ 19
4.3 Water Sampling .......................................... 20
4.4 Other Sampling .......................................... 20
5.0 REQUEST FOR ANALYSES ..................................... 23
5.1 Analyses Narrative ........................................ 23
5.2 Analytical Laboratory ...................................... 23
6.0 FIELD METHODS AND PROCEDURES ............................. 29
6.1 Field Equipment .......................................... 29
6.1.1 List of Equipment Needed ............................. 29
6.1.2 Calibration of Field Equipment ......................... 29
6.2 Field Screening ........................................... 29
6.3 Soil ................................................... 30
6.3.1 Surface Soil Sampling ................................ 30
6.3.2 Subsurface Soil Sampling ............................. 31
6.4 Sediment Sampling ........................................ 33
6.5 Water Sampling .......................................... 33
6.5.1 Surface Water Sampling .............................. 33
6.5.2 Groundwater Sampling .............................. 35
6.6 Other ................................................. 39
6.7 Decontamination Procedures ................................. 39
SAP general 5 12/05/2011
7.0 SAMPLE CONTAINERS, PRESERVATION, PACKAGING AND SHIPPING .. 43
7.1 Soil Samples ............................................. 43
7.2 Sediment Samples ......................................... 44
7.3 Water Samples ........................................... 44
7.4 Other Samples ........................................... 45
7.5 Packaging and Shipping ..................................... 46
8.0 DISPOSAL OF RESIDUAL MATERIALS ............................ 48
9.0 SAMPLE DOCUMENTATION AND SHIPMENT ....................... 51
9.1 Field Notes .............................................. 51
9.1.1 Field Logbooks .................................... 51
9.1.2 Photographs ...................................... 52
9.2 Labeling ............................................... 52
9.3 Sample Chain-Of-Custody Forms and Custody Seals ................ 53
10.0 QUALITY CONTROL ......................................... 54
10.1 Field Quality Control Samples ............................... 54
10.1.1 Assessment of Field Contamination (Blanks) ............... 54
10.1.2 Assessment of Field Variability (Field Duplicate or Co-located
Samples .............................................. 58
10.2 Background Samples ...................................... 60
10.3 Field Screening, Confirmation and Split Samples .................. 60
10.3.1 Field Screening Samples ............................. 60
10.3.2 Confirmation Samples .............................. 61
10.3.3 Split Samples ..................................... 61
10.4 Laboratory Quality Control Samples .......................... 62
11.0 FIELD VARIANCES .......................................... 64
12.0 FIELD HEALTH AND SAFETY PROCEDURES ...................... 65
SAP general 6 12/05/2011
ATTACHMENT 1 - EXAMPLE FORMS
Table 1-1: Key Project Personnel Contact Information and Responsibilities
Table 2-1: Contaminants of Concern – Previous Investigations, Matrix = Soil
Table 3-1: Contaminants of Concern, Laboratory and Action Levels, Matrix = Soil
Table 4-1: Sampling Design and Rationale, Matrix = Soil
Table 4-2: Sampling Design and Rationale, Matrix = Groundwater
Table 5-1: Analytical Services, Matrix = Soil
Table 5-2: Analytical Services, Matrix = Groundwater
Table 5-3: Analytical Method, Containers, Preservation, and Holding Times
Requirements, Matrix = Soil
Table 5-4: Analytical Method, Containers, Preservation, and Holding Times
Requirements, Matrix = Groundwater
Table 6-1: Field and Sampling Equipment
Table 6-2: Field Equipment/Instrument Calibration, Maintenance, Testing, and Inspection

SAP general 7 12/05/2011
1.0 INTRODUCTION
This section should include a brief description of the project, the problem to be investigated, and
the scope of sampling effort. These topics will be covered in depth later so do not include a
detailed discussion here.
1.1 Site Name or Sampling Area
Provide the most commonly used name of the site or sampling area. Also include the name or
abbreviation (e.g., “the Site”), if any, that will be used throughout the plan.
1.2 Site or Sampling Area Location
Provide a general description of the region, state or tribal area in which the site or sampling
area is located. Include the street address, city, state, and postal code, if appropriate. Detailed
sampling location information should be provided later in Section 2.
1.3 Responsible Agency
Provide a description of the organization conducting the sampling.
1.4 Project Organization
Fill in the information requested in Table 1-1, modifying it to the specific project and deleting
what is not relevant. Provide the name, phone number, and email address of the person(s)
and/or contractor(s) working on the sampling project as listed in the table. A brief description
of the roles and responsibilities for each key position should be included, either in the table (as
shown) or within the text of this section. An Organization Chart should be included showing the
lines of communication.
It should be noted that it is the responsibility of the Quality Assurance (QA) Officer to oversee
the implementation of the Sampling and Analysis Plan (or QA Project Plan if one has been
prepared), including whether specified quality control (QC) procedures are being followed as
described. Ideally, this individual should discuss QA issues with the Project Manager, but
should not be involved in the data collection/analysis/interpretation/reporting process except in
a review or oversight capacity. If the project is small, another technical person may fulfill this
role.

SAP general 8 12/05/2011
Table 1-1 – Key Project Personnel Contact Information and Responsibilities
Title
Name
Phone Number
Email Address
Responsibilities
EPA Project Manager
EPA Quality Assurance
Officer (QAO)
Grantee Project Manager
Contractor Project
Manager (include
Company Name)
Contractor QAO
Contractor Field Team
Leader
Laboratory Quality
Assurance Officer (include
Laboratory Name)

SAP general 9 12/05/2011
2.0 BACKGROUND
This section provides an overview of the location, previous investigations, and the apparent
problem(s) associated with the site or sampling area.
2.1 Site or Sampling Area Description
At a minimum, two maps of the area should be provided: the first should place the area within its
geographic region (i.e., State); the second, on a smaller scale, should mark the sampling site or
sampling areas within the local area. Additional maps may be provided, as necessary, for
clarity. Maps should include a North arrow, a surface and/or ground water directional flow
arrow (if appropriate), buildings or former buildings, spill areas, etc. If longitude or latitude
information is available, such as from a Global Positioning System (GPS), provide it.
Fill in the blanks.
The site or sampling area occupies __________ [e.g., acres or square feet] in a
________________ [e.g., urban, commercial, industrial, residential, agricultural, or
undeveloped] area. The site or sampling area is bordered on the north by ___________, on the
west by ______________, on the south by ________________, and on the east by
________________. The specific location of the site or sampling area is shown in Figure ____.
Additional information should be provided that describes the historic and current on-site
structures and other important site features. These should be shown on one of the figures.
2.2 Operational History
As applicable, describe in as much detail as needed the past and present activities at the site or
sampling area. The discussion might include the following information:
• a description of the owner(s) and/or operator(s) of the site or areas near the site or the
sampling area (in chronological order);
• a description of past and current operations or activities that may have contributed to
suspected contamination;
• a description of the processes involved in the operation(s) and the environmentally
detrimental substances, if any, used in the processes;
• a description of any past and present waste management practices.

SAP general 10 12/05/2011
2.3 Previous Investigations/Regulatory Involvement
Summarize all previous sampling efforts at the site or sampling area, including the:
• sampling date(s);
• name of the party(ies) that conducted the sampling;
• local, tribal, state or federal government agency for which the sampling was conducted;
• a rationale for the sampling;
• the type of media sampled (e.g., soil, sediment, water);
• laboratory methods that were used;
• a discussion of what is known about data quality and usability.
The summaries should be presented in subsections chronologically. Attach reports or summary
tables of results, or include in appendices. See Table 2-1 for an example. Previous sampling
locations can be shown on one of the figures or in additional figures.
2.4 Geological Information
For surface and/or ground water sampling: Provide a description of the hydrogeology of the
area. Indicate the direction of flow for surface and/or ground water and include a directional
flow arrow on the appropriate figure.
For soil sampling: Provide a description of the geology of the area.
For air sampling: Provide prevailing wind direction, temperature, etc.
2.5 Environmental and/or Human Impact
Discuss what is known about the potential and actual impacts of the possible environmental
problem at the site on human health or the environment.

SAP general 11 12/05/2011
Table 2-1: Contaminants of Concern – Previous Investigations
(Matrix = xx)
Analytical Parameter
(Contaminants of Concern)
Date of sampling
Sampling contractor
Laboratory
Analytical Results
(units)
Regulatory Limit
(specify)
1
1
Specify the source of the regulatory limit(s). For example:
DTSC = Calif. Department of Toxic Substances Control
RWQCB = Regional Water Quality Control Board
PRGs = Preliminary Remediation Goal (2004)
CHHSLs = California Human Health Screening Levels
ESLs = Environmental Screening Levels

SAP general 12 12/05/2011
3.0 PROJECT DATA QUALITY OBJECTIVES
3.1 Project Objectives and Problem Definition
Describe the purpose of the environmental investigation and how the data will be used. Discuss
how the site’s history relates to the problem to be investigated, the scope of the sampling effort,
and the types of analyses required. Include all measurements to be made on an analyte-specific
basis in whatever media (soil, sediment, water, etc.) are to be sampled. This discussion should
relate the sampling effort to the specific decisions described below in Section 3.2.
3.2 Data Quality Objectives (DQOs)
Data quality objectives (DQOs) are quantitative and qualitative criteria upon which project
decisions are based. DQOs should cover the following items:
Describe the problem to be investigated.
Identify what questions the study will attempt to answer, what actions (decisions) may
result, and who the primary decision maker is.
Identify the information that needs to be obtained and the measurements that need to be
taken to resolve the decision statement(s).
Define study boundaries, and when and where data should be collected.
The use of “...if...then” statements is recommended. Note that decisions do not have to involve
regulatory or legal action. Some examples: “If contaminants of concern are not detected above
the action level, then no further action is required.” or “If contaminants of concern are found
above the action level, then recommendations for further action, such as additional sampling,
will be evaluated.”
This section should describe decisions to be made based on the data and provide criteria on
which these decisions will be made. Inclusion of one or more tables is recommended. See Table
3-1 for reference. A separate table should be prepared for each matrix/media to be sampled.
Tables should contain, at a minimum, the main contaminants of concern, their associated action
levels and detection limits, and the source of the action level (regulation, health based criteria,
water quality standards, etc.) If a contaminant does not have an action level, or will not be used
in decision making, the text should discuss how the data for that contaminant will be used.

SAP general 13 12/05/2011
As projects utilizing this template are limited in scope, defining action levels and measurement
quality objectives (MQOs) for field and laboratory measurements should be sufficient. The
project manager, or other decision maker identified earlier in the project organization section,
must determine what level of uncertainty is acceptable.
More sophisticated DQO discussions involve defining null test hypotheses and confidence
intervals. These should be considered depending on project decision making needs, but
generally such discussions are not expected to be applicable in limited sampling events. EPA’s
Guidance for Systematic Planning Using the Data Quality Objectives Process (EPA QA/G-4,
February 2006) should be consulted for more information.
In addition to meeting defined DQOs, data quality is also evaluated as to its conformance to
measurement quality objectives (MQOs), which are discussed in the next section.
3.3 Data Quality Indicators (DQIs) and Measurement Quality Objectives (MQOs)
Data Quality Indicators (DQIs) provide a means to evaluate the quality of data and are normally
defined in terms of PARCCS (precision, accuracy, representativeness, completeness,
comparability, and sensitivity (method detection limits). Precision, accuracy, and sensitivity are
usually covered in method specific criteria (see below). However, the other DQIs
(representativeness, completeness, and comparability) should be defined in the plan for the
project as a whole.
The values that are to be assigned to the quantitative data quality indicators (accuracy,
precision, completeness and sensitivity) and statements concerning the qualitative indicators
(representativeness and comparability) are determined by the answers to the question: How
sure are you that the values of the data are what the analyses have determined them to be?
Each DQI needs to have defined data quality acceptance criteria (measurement quality
objectives (MQOs), as well as a means for assessing whether the criteria were achieved.
Whenever possible, it is desirable that the MQOs be expressed in numerical or quantitative
terms, along with one or more associated quality control (QC) samples that will serve as a
means for assessing the DQI.
All the elements of the sampling event, from the sampling design and collection through
laboratory analysis and reporting, affect the quality of the data. Depending on what the
contaminants of concern are, what effect they may have on human and environmental health,

SAP general 14 12/05/2011
and at what level, uncertainty around a data point may need to be legally defensible at one
extreme or only providing the answer to the “presence-absence” question at the other.
Measurement quality objectives (MQOs) can be defined in one of three ways: by the analytical
methods (often referred to as method performance criteria (MPCs)); by the laboratory; or by the
project. Laboratory or project defined MQOs typically result when an analytical method has
been modified to meet laboratory capabilities or project needs.
Where applicable, precision and accuracy limits for both laboratory and field measurements
may be presented in a table. See Tables 3-2 and 3-3 for examples. A separate table should be
prepared for each matrix or media to be sampled. If not presented as tables in the text, MQO
tables or laboratory SOPs should be included as appendices and referenced. This is discussed in
greater detail in Section 5.2.
Definitions of the PARCCS terms are provided below:
Precision is the degree of mutual agreement between or among independent
measurements of a similar property (usually reported as a standard deviation [SD] or
relative percent difference [RPD]). This indicator relates to the analysis of duplicate
laboratory or field samples. Typically, field precision is assessed by co-located samples,
field duplicates, or field splits and laboratory precision is assessed using laboratory
duplicates, matrix spike duplicates, or laboratory control sample duplicates.
Accuracy is the degree of agreement of a measurement with a known or true value and
includes a combination of the random error (precision) and the systematic error (bias)
components of both sampling and analytical operations. Given the nature of projects
using the Template, only the bias component is considered. To determine accuracy, a
laboratory or field value is compared to a known or true concentration. Accuracy is
determined by such QC indicators as: matrix spikes, surrogate spikes, laboratory control
samples (blank spikes) and performance samples.
Representativeness is the expression of the degree to which data accurately and precisely
represent a characteristic of an environmental condition or a population. It relates both
to the area of interest and to the method of taking the individual sample. The idea of
representativeness should be incorporated into discussions of sampling design.
Representativeness is best assured by a comprehensive statistical sampling design, but it
is recognized that this is usually outside the scope of most one-time events. Most one-

SAP general 15 12/05/2011
time SAPs should focus on issues related to judgmental sampling and why certain areas
are included or not included and the steps being taken to avoid either false positives or
false negatives.
Completeness is expressed as percent of valid usable data actually obtained compared to
the amount that was expected. It may happen that, due to a variety of circumstances,
either not all samples scheduled to be collected were collected or else the data from
samples cannot be used due to, for example, loss or spillage of samples, instrument
failures, technical mistakes, etc.. The minimum percent of completed analyses defined in
this section depends on how much information is needed for decision making. Generally,
the fewer the number of samples taken per event or the more critical the data are for
decision making, the higher the completeness goals. Goals in the 75-90% range are
typical.
Comparability expresses the confidence with which one data set can be compared to
another. The use of generally accepted and published methods from a recognized source,
such as EPA (i.e., methods listed in http://www.epa.gov/waterscience/methods/method)
or Standard Methods for the Examination of Water and Wastewater allows the data to be
compared to similar data sets, facilitating evaluation of trends or changes in a site, a
river, groundwater, etc. Comparability also refers to the reporting of data in comparable
units.
Sensitivity, usually expressed as method detection limits (MDLs) or quantitation limit for
all analytes or compounds of interest for all analyses requested must be included in this
section. These limits should be related to any decisions that will be made as a result of
the data collection effort. A critical element to be addressed is how these limits relate to
any regulatory or action levels that may apply.
3.4 Data Review and Validation
Discuss data review and data validation, including what organizations or individuals will be
responsible for what aspects of data review and what the review will include. This section
should also discuss how data that do not meet data quality objectives will be designated, flagged,
or handled. Possible corrective actions associated with the rejection of data, such as reanalysis
or resampling, should be addressed.

SAP general 16 12/05/2011
Region 9 has adopted a tiered approach to data review. Details on validation are available from
the QA Office, but a brief summary follows:
Tier 1 involves a general review of the QC data for the project. This is sometimes
referred to as a “Summary Forms” review. At a minimum, all data should receive a Tier
1 review.
Tier 2 involves a selected validation of a portion of the data. Which aspect of the project
is to be reviewed should be defined in the DQO discussion of the project. The focus
might be on a specific area within the sampling area, specific analytes or analyses of
concern critical to decision making, or some other factor(s). The review may also look at
unusual results noted in the Tier 1 review.
Tier 3 involves validation of all the data collected and reported. This includes a review
of the raw data, the laboratory’s standards log books, extractions logs, instrument
printouts, chromatograms (if applicable), mass spectra (if applicable), etc. Calibration
data, sample analysis data, and quality control data are all evaluated. Typically, this is a
“third party review” and is based on strict protocols, such as the National Functional
Guidelines (http://www.epa.gov/superfund/programs/clp/download/fgorg.pdf).
It is recommended that if validation will be a part of the data review process, that SOP(s) from
the organization which will perform the validation be attached.
3.5 Data Management
Provide a list of the steps that will be taken to ensure that data are transferred accurately from
collection to analysis to reporting. Discuss the measures that will be taken to review the data
collection processes, including field notes or field data sheets; to obtain and review complete
laboratory reports; and to review the data entry system, including its use in reports. A checklist
is acceptable.
3.6 Assessment Oversight
Describe the procedures which will be used to implement the QA Program. This includes
oversight by the Quality Assurance Manager or the person assigned QA responsibilities.
Indicate how often a QA review of the different aspects of the project, including audits of field
and laboratory procedures, use of performance samples, review of laboratory and field data,

SAP general 17 12/05/2011
etc., will take place. Describe what authority the QA Manager or designated QA person has to
ensure that identified field and analytical problems will be corrected and the mechanism by
which this will be accomplished and documented.

SAP general 18 12/05/2011
Table 3-1: Contaminants of Concern, Laboratory and Action Levels
(Matrix = xx)
Analytical Parameter
(Contaminants of Concern)
Laboratory
Reporting or
Quantitation
Limits
Action Levels

SAP general 19 12/05/2011
4.0 SAMPLING RATIONALE
For each sampling event, the SAP must describe the sampling locations, the media to be
sampled, and the analytes of concern at each location. A rationale should be provided to
support these choices. The information may be presented in a table. See Tables 4-1 and 4-2 for
examples.
The following subsections, as applicable, must be included for plan approval and should be
consistent with the project DQOs. They are subdivided on a media specific basis (soil, sediment,
and water). Other media should be added as needed. Appropriate figures should be included
showing proposed sampling locations.
Information regarding the collection of field duplicates may be included in these sections or in
Section 10.1.2. Provide a rationale for the selection of these locations.
Do not include sampling procedures, preservation, etc., as these topics are covered in later
sections.
4.1 Soil Sampling
Provide a general overview of the soil sampling event. Present a rationale for choosing each
sampling location at the site or sampling area and the depths at which the samples are to be
taken, if relevant. If decisions will be made in the field, provide details concerning the criteria to
be used to make these decisions. List the analytes of concern at each location and provide a
rationale as to why the specific chemical or group of chemicals was chosen. Include a figure
showing sampling locations.
4.2 Sediment Sampling
Provide a general overview of the sediment sampling event. Present a rationale for choosing
each sampling location at the site or sampling area and the depths or area of the river, stream or
lake at which the samples are to be taken, if relevant. If decisions will be made in the field,
provide details concerning the criteria to be used to make these decisions. List the analytes of
concern at each location and provide a rationale as to why the specific chemical or group of
chemicals was chosen. Include a figure showing sampling locations.

SAP general 20 12/05/2011
4.3 Water Sampling
Provide a general overview of the water sampling event. For groundwater, describe the wells to
be sampled or how the samples will be collected (e.g., hydro punch), including the depths at
which the samples are to be taken. For surface water, describe the depth and nature of the
samples to be collected (fast or slow moving water, stream traverse, etc.). Present a rationale
for choosing each sampling location or sampling area. If decisions will be made in the field,
provide details concerning the criteria to be used to make these decisions. List the analytes of
concern at each location and provide a rationale as to why the specific chemical or group of
chemicals was chosen. Include a figure showing sampling locations.
4.4 Other Sampling
Describe other media, if any, that may be sampled. Present a rationale for choosing each
sampling location at the site or sampling areas, and the depths at which the samples will be
taken, if relevant. If decisions will be made in the field, provide details concerning the criteria to
be used to make these decisions. List the analytes of concern at each location and provide a
rationale as to why the specific chemical or group of chemicals was chosen. Include a figure
showing sampling locations.

SAP general 21 12/05/2011
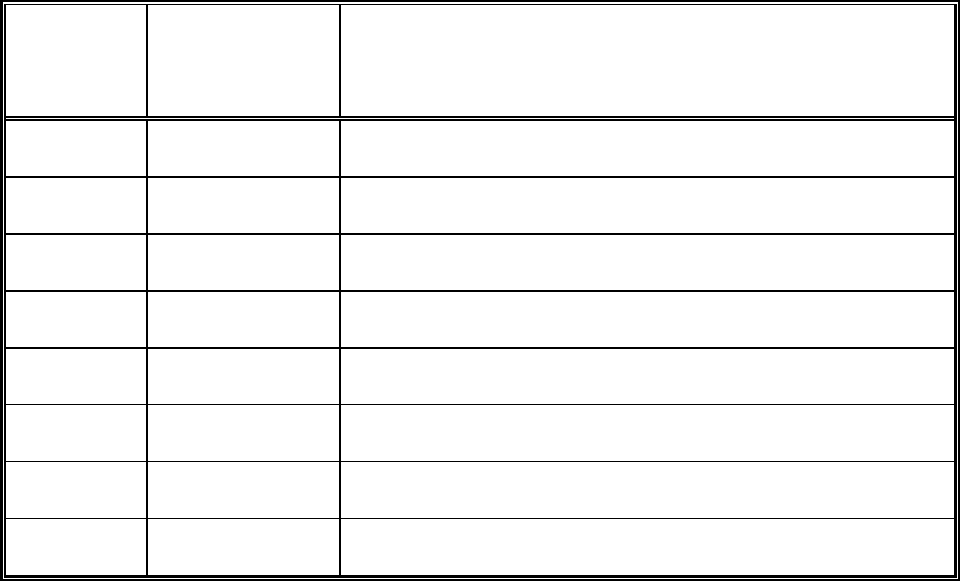
Table 4-1: Sampling Design and Rationale
Matrix = Soil
Sampling
Location/ID
Number
Depth
(ft)
Analytical
Parameter
Rationale *
* Include rationale for location, depth and analysis.
SAP general 22 12/05/2011
Table 4-2: Sampling Design and Rationale
Matrix = Groundwater
Sampling
Location/ID
Number
Analytical
Parameter
Rationale *
* Include rationale for location and analysis.

SAP general 23 12/05/2011
5.0 REQUEST FOR ANALYSES
The following sections should discuss the analytical support for the project: the analyses
requested; analytes of concern; turnaround time;, available resources; available laboratories;
etc. The use of tables is recommended. If samples will be sent to more than one laboratory, it
should be clear where each sample is to be sent.
5.1 Analyses Narrative
Complete this subsection concerning the analyses for each matrix. The use of an analytical
services table is recommended for each matrix to be sampled. See Tables 5-1 and 5-2 for
examples. Each table must include the analytical parameters for each type of sample. Quality
Control (QC) samples, such as blanks, duplicates, splits, and laboratory QC, should be included
in the column titled “Special Designation.” The selected analyses must be consistent with the
DQOs and analytes of concern.
Information on container types, sample volumes, preservatives, special handling and analytical
holding times for each parameter may be included here or on separate tables. See Tables 5-3
and 5-4 for examples.
Include any special requests, such as fast turn-around time (2 weeks or less), specific QC
requirements, or modified sample preparation techniques in this section.
Note: Rationale for the selection of duplicate and laboratory QC sample locations is to be
provided in Section 10.0.
5.2 Analytical Laboratory
When an organization contracts for analytical work it has two options. In Option 1, MQOs for
laboratory work are defined in the SAP. The MQOs are provided to the laboratory which states
whether it is capable of meeting these criteria and that it is willing to do so. In Option 2, the
sampling organization reviews the laboratory’s information about its QA/QC Program and
Criteria and determines whether the laboratory can meet project needs.
If the first approach is taken, the organization writing the SAP should include the relevant QC
tables in the SAP. The Region 9 QA Office has MQO tables available for most routine analyses.
These tables can be attached to the SAP and referenced in this section. Plan preparers can

SAP general 24 12/05/2011
request these tables, review them for project appropriateness, and incorporate all or some of
them as is or in a modified form into the SAP.
If the second approach is taken, the sampling organization must acknowledge that it understands
and agrees to the MQOs defined by the contract laboratory. MQOs or QC criteria for work
performed by the laboratory will be found in either the laboratory’s QA Plan and/or its SOPs,
which must be included with the SAP for EPA QA Office review.
If analytical analyses are arranged through the Region 9 Analytical Services (RAS), this should
be stated. Analytical options include the Contract Laboratory Program Analytical Services
(CLPAS) (Superfund and Brownfields projects only), the Region 9 Laboratory in Richmond,
California, and commercial laboratories under contract to Region 9. The Regional Sample
Control Coordinator (RSCC) determines where samples will be sent. The SAP should not
contain this information unless the RSCC has indicated in advance where the samples will be
sent. If samples will be sent to laboratories through RAS and also to another laboratory with
which the sampling organization has established a contract, this section should make it clear
which samples go to each laboratory.
Field analyses for pH, conductivity, turbidity, or other field tests should be discussed in the
sampling section. Field measurements in a mobile laboratory should be discussed here and
listed separately from samples to be sent to a fixed laboratory. Field screening tests (for
example, immunoassay tests) should be discussed in the sampling section, but the confirmation
tests should be discussed here and the totals included in the tables.
The narrative subsection concerning laboratory analytical requirements should be completed.
Appropriate MQO tables or the laboratory QA Plan and relevant SOPs for the methods to be
performed must accompany the SAP. Although EPA does not approve or certify laboratories,
the QA Office will review the laboratory’s QA Plan and provide comments to the SAP’s
originator concerning whether the laboratory’s QA/QC program appears to be adequate to meet
project objectives. It is recommended that any issues raised be discussed with the laboratory
and resolved before work commences. Note that the more the SAP “defaults” to laboratory
capabilities, the greater emphasis will be placed on the adequacy of the laboratory’s QA
program. If the SAP author generates MQO tables or the equivalent for the project, the
laboratory will be required to state clearly whether its program is sufficient to meet the project’s
analytical objectives.

SAP general 25 12/05/2011
If samples will be sent through the Regional Analytical Services (RAS) system, the SAP should
include, at a minimum, a table containing the compounds of concern and their respective
detection limits. (See Section 3.2.)

SAP general 26 12/05/2011
Table 5-1: Analytical Services
Matrix = Soil
Sample
Number
Sample
Location
Depth
(ft)
Special
Designation
Analytical Methods
Total number of Soil Samples, excluding QC:
Total number of Soil Samples, including QC:

SAP general 27 12/05/2011
Table 5-2: Analytical Services
Matrix = Groundwater
Sample
Number
Sample
Location
Special
Designation
Analytical Methods
Total number of samples, excluding QC
Total number of samples, including QC

SAP general 28 12/05/2011
Table 5-3: Analytical Method, Containers, Preservation,
and Holding Times Requirements
Matrix = Soil
Analytical
Parameter
and/or Field
Measurements
Analytical
Method Number
Containers
(number, type,
size/volume)
Preservation
Requirements
(chemical,
temperature,
light protection)
Maximum
Holding Times
Table 5-4: Analytical Method, Containers, Preservation,
and Holding Times Requirements
Matrix = Groundwater
Analytical
Parameter
and/or Field
Measurements
Analytical
Method Number
Containers
(number, type,
size/volume)
Preservation
Requirements
(chemical,
temperature,
light protection)
Maximum
Holding Times

SAP general 29 12/05/2011
6.0 FIELD METHODS AND PROCEDURES
The sampling discussion should track the samples identified in Section 4.0 and the Analytical
Services table(s). Provide a description of sampling procedures. Example procedures are
provided below, but if they exist, the organization’s procedures should be described instead. If
that is the case, attach a copy of the applicable SOP. Some sampling procedures are available
from EPA. Contact the QA Office or visit the Region 9 laboratory’s web page. A general
statement should be made that refers to the sections containing information about sample
tracking and shipping (Section 7).
Depending on the nature of the project, some of the following sections may not be applicable. If
this is the case, enter “Not Applicable” or other text to indicate that the section does not apply.
6.1 Field Equipment
6.1.1 List of Equipment Needed
List all the equipment to be used in the field to collect samples, including decontamination
equipment, if required. Discuss the availability of back-up equipment and spare parts. This
information can be presented in a table. See Table 6-1 for an example.
6.1.2 Calibration of Field Equipment
Describe the procedures by which field equipment is prepared for sampling, including
calibration standards used, frequency of calibration and maintenance routines. Indicate where
the equipment maintenance and calibration record(s) for the project will be kept. See Table 6-2
for an example.
6.2 Field Screening
In some projects, a combination of field screening using a less accurate or less sensitive method
and confirmation samples to be analyzed in a fixed laboratory will be used. This section should
describe the field methods or reference attached SOPs. Analyses such as XRF or immunoassay
kits are two examples.
Describe how samples will be collected, prepared, and analyzed in the field. Include in an
appendix any SOPs relating to these methods. Confirmation of screening results should also be

SAP general 30 12/05/2011
described. The role of the field screening in decision making for the site should be discussed
here if it has not been covered previously.
6.3 Soil
6.3.1 Surface Soil Sampling
Use this subsection to describe the surface soil samples that are to be collected within 6-12
inches of the ground surface. Specify the method (e.g., hand trowels) that will be used to collect
and transfer the samples to the appropriate containers, or reference the appropriate sections of
a Soil Sampling SOP. If SOPs are referenced, they should be included in an appendix.
If exact soil sampling locations will be determined in the field, this should be stated. The criteria
used to determine sampling locations should be provided.
Include this paragraph first if exact sampling locations are to be determined in the field;
otherwise delete.
Exact soil sampling locations will be determined in the field based on accessibility, visible signs
of potential contamination (e.g., stained soils), and topographical features which may indicate the
location of hazardous substance disposal (e.g., depressions that may indicate a historic
excavation). Soil sample locations will be recorded in the field logbook as sampling is
completed. A sketch of the sample location will be entered into the logbook and any physical
reference points will be labeled. If possible, distances to the reference points will be given.
It is Region 9 policy that soils collected for volatile analysis be collected in hermetically sealed
sampling devices such as EnCore samplers and analyzed within the holding time specified in
EPA Method 5035, or immediately preserved by one of the processes specified in EPA Method
5035. Collection in brass tubes, even if preserved, is not acceptable. Hermetically sealed
sampling devices are also required for gasoline samples. A rationale should be provided if more
than one preservation method is specified. (Note: Preservation with methanol may significantly
increase detection limits.)
If surface soil samples are to be analyzed for volatile organic compounds (VOCs), include this
paragraph; otherwise delete.

SAP general 31 12/05/2011
Samples to be analyzed for volatile organic compounds will be collected first. Surface soil
samples for VOC analyses will be collected as grab samples (independent, discrete samples)
from a depth of 0 to ___ inches below ground surface (bgs). Surface soil samples will be
collected using [specify the type of sampling device], and will be collected in triplicate. Sample
contianers will be sealed and placed in a zip lock bag. See Section 7.1 for preservation and
shipping procedures.
If surface soil samples are to be analyzed for compounds other than volatiles, include this
paragraph; otherwise delete.
Surface soil samples will be collected as grab samples (independent, discrete samples) from a
depth of 0 to ___inches below ground surface (bgs). Surface soil samples will be collected using
a stainless steel hand trowel. Samples to be analyzed for __________ [list all analytical methods
for soil samples except volatile organic compounds] will be placed in a sample-dedicated
disposable pail and homogenized with a trowel. Material in the pail will be transferred with a
trowel from the pail to the appropriate sample containers. Sample containers will be filled to the
top, taking care to prevent soil from remaining in the lid threads prior to being closed to prevent
potential contaminant migration to or from the sample. [Alternatively, samples will be retained
in the brass sleeves in which collected until sample preparation begins.] See Section 7.1 for
preservation and shipping procedures.
6.3.2 Subsurface Soil Sampling
Use this subsection for subsurface soil samples that are to be collected 12 inches or more below
the surface. Specify the method (e.g., hand augers) that will be used to reach the appropriate
depth, state the depth at which samples will be collected and describe the method used to collect
and transfer samples to the appropriate containers or reference the appropriate sections of a
Soil Sampling SOP. If SOPs are referenced, they should be included in an Appendix.
If exact soil sampling locations will be determined in the field, this should be stated. The criteria
used to determine sampling locations should be provided. There should also be a discussion
concerning possible problems, such as subsurface refusal.
Include this paragraph first if exact sampling locations are to be determined in the field;
otherwise delete.

SAP general 32 12/05/2011
Exact soil sampling locations will be determined in the field based on accessibility, visible signs
of potential contamination (e.g., stained soils), and topographical features which may indicate the
location of hazardous substance disposal (e.g., depressions that may indicate a historic
excavation). Soil sample locations will be recorded in the field logbook as sampling is
completed. A sketch of the sample location will be entered into the logbook and any physical
reference points will be labeled. If possible, distances to the reference points will be given.
It is Region 9 policy that soils collected for volatile analysis be collected in hermetically sealed
sampling devices. See the discussion in Section 6.3.1.
If subsurface soil samples are to be analyzed for volatile organic compounds, include this
paragraph; otherwise delete.
Samples to be analyzed for volatile organic compounds (VOCs) will be collected first.
Subsurface samples will be collected by boring to the desired sample depth using
____________________. Once the desired sample depth is reached, soil samples for VOC
analyses will be collected as independent, discrete samples. Samples will be collected using
[specify the type of sampling device], and will be collected in triplicate. Samples will be sealed
and placed in a zip lock bag. See Section 7.1 for preservation and shipping procedures.
If subsurface soil samples are being collected for compounds other than volatiles, include this
paragraph; otherwise delete.
Subsurface samples will be collected by boring to the desired sample depth using ____________
____________________. Once the desired sample depth is reached, the ___________________
_______________ [hand- or power-operated device, such as a shovel, hand auger, trier, hollow-
stem auger or split-spoon sampler] will be inserted into the hole and used to collect the sample.
Samples will be transferred from the _____________________ [sampling device] to a sample-
dedicated disposable pail and homogenized with a trowel. Material in the pail will be transferred
with a trowel from the pail to the appropriate sample containers. Sample containers will be filled
to the top taking care to prevent soil from remaining in the lid threads prior to being sealed to
prevent potential contaminant migration to or from the sample. See Section 7.1 for preservation
and shipping procedures.
Include this statement for all types of analyses.
Excess set-aside soil from the above sampled interval will then be repacked into the hole.

SAP general 33 12/05/2011
6.4 Sediment Sampling
Use this subsection if sediment samples are to be collected. Specify the method (e.g., dredges)
that will be used to collect the samples and at what depth samples will be collected. Describe
how samples will be homogenized and transferred to the appropriate containers. If an SOP will
be followed, it should be referenced and included in the appendix.
If exact sediment sampling locations will be determined in the field, this should be stated.
Describe where sediment samples will be collected
Exact sediment sampling locations will be determined in the field, based on ____________
__________________. [Describe the criteria to be used to determine sampling locations.] Care
will be taken to obtain as representative a sample as possible. The sample will be taken from
areas likely to collect sediment deposits, such as slow moving portions of streams or from the
bottom of the lake at a minimum depth of ___ feet.
The final paragraph describes sample homogenization, which is particularily important if the
sample is to be separated into solid and liquid phases, and how the container is to be filled.
Include this paragraph for all sediment sampling. It is assumed that sediment samples will not
be analyzed for volatile compounds. However, if sediment is to be analyzed for volatile organic
compounds, the samples to be analyzed for volatile compounds should not be homogenized, but
rather transferred directly from the sampler into the sample container. If feasible, a
hermetically sealed sampling device should be used.
Material in the sampler will be transferred to a sample-dedicated one gallon disposable pail and
homogenized with a trowel. Material from the pail will be transferred with a clean trowel from
the bucket to the appropriate sample containers. Sample containers will be filled to the top,
taking care to prevent soil from remaining in the lid groves prior to being sealed in order to
prevent potential contamination migration to or from the sample containers. See Section 7.2 for
preservation and shipping procedures.
6.5 Water Sampling
6.5.1 Surface Water Sampling
SAP general 34 12/05/2011
Use this subsection if samples are to be collected in rivers, streams, lakes and reservoirs, or
from standing water in runoff collection ponds, gullies, drainage ditches, etc. Describe the
sampling procedure, including the type of sample (grab or composite - see definitions below),
sample bottle preparation, and project-specific directions for taking the sample. State whether
samples will be collected for chemical and/or microbiological analyses. Alternatively, reference
the appropriate sections of attached SOPs.
Grab: Samples will be collected at one time from one location. The sample should be
taken from flowing, not stagnant water, and the sampler should be facing upstream in the
middle of the stream. Samples will be collected by hand or with a sample bottle holder.
For samples taken at a single depth, the bottle should be uncapped and the cap protected
from contamination. The bottle should be plunged into the water, mouth down, and filled
6 to 12 inches below the surface of the water. If it is important to take samples at depths,
special samplers (e.g., Niskin or Kemmerer Depth Samplers) may be required.
Time Composite: Samples are collected over a period of time, usually 24 hours. If a
composite sample is required, a flow- and time-proportional automatic sampler should
be positioned to take samples at the appropriate location in a manner such that the
sample can be held at 4
o
C for the duration of the sampling.
Spatial Composite: Samples are collected from different representative positions in the
water body and combined in equal amounts. A Churn Splitter or equivalent device will
be used to ensure that the sample is homogeneously mixed before the sample bottles are
filled. Volatile organic compound samples will be collected as discrete samples and not
composited.
If exact surface water sample locations will be determined in the field, this should be stated.
Describe the criteria used to determine where surface water samples will be collected.
Include this paragraph first if exact sampling locations are to be determined in the field;
otherwise delete.
Exact surface water sampling locations will be determined in the field based on __________
____________________ [describe the criteria to be used to determine sampling locations].
Sample locations will be recorded in the field logbook as sampling is completed. A sketch of the
sample location will be entered into the logbook and any physical reference points will be
labeled. If possible, distances to the reference points will be provided.

SAP general 35 12/05/2011
Use this paragraph if samples are to be collected in rivers, streams, lakes and reservoirs, or
from standing water in runoff collection ponds, gullies, drainage ditches, etc.
Samples will be collected from _____________ [describe the sampling location]. [Describe the
sampling procedure (e.g., grab, time composite, spatial composite), sample bottle preparation,
and project-specific directions for taking the sample, or reference the appropriate sections of a
Water Sampling SOP. If SOPs are referenced, they should be included in an appendix.] See
Section 7.3 for preservation and shipping procedures.
6.5.2 Groundwater Sampling
This subsection contains procedures for water level measurements, well purging, and well
sampling. Relevant procedures should be described under this heading with any necessary site-
specific modifications. Alternatively, reference appropriate SOP(s).
6.5.2.1 Water-Level Measurements
The following language may be used as is or modified to meet project needs.
All field meters will be calibrated according to manufacturer's guidelines and specifications
before and after every day of field use. Field meter probes will be decontaminated before and
after use at each well.
If well heads are accessible, all wells will be sounded for depth to water from top of casing and
total well depth prior to purging. An electronic sounder, accurate to the nearest +/- 0.01 feet,
will be used to measure depth to water in each well. When using an electronic sounder, the
probe is lowered down the casing to the top of the water column. The graduated markings on the
probe wire or tape are used to measure the depth to water from the surveyed point on the rim of
the well casing. Typically, the measuring device emits a constant tone when the probe is
submerged in standing water and most electronic water level sounders have a visual indicator
consisting of a small light bulb or diode that turns on when the probe encounters water. Total
well depth will be sounded from the surveyed top of casing by lowering the weighted probe to
the bottom of the well. The weighted probe will sink into silt, if present, at the bottom of the
well screen. Total well depths will be measured by lowering the weighted probe to the bottom of
the well and recording the depth to the nearest 0.1 feet.

SAP general 36 12/05/2011
Water-level sounding equipment will be decontaminated before and after use in each well.
Water levels will be measured in wells which have the least amount of known contamination
first. Wells with known or suspected contamination will be measured last.
6.5.2.2 Purging
Describe the method that will be used for well purging (e.g., dedicated well pump, bailer, hand
pump), or reference the appropriate sections in a Ground Water SOP. If SOPs are referenced,
they should be included in an appendix. Note: A combination of purging methods may be used.
Include this paragraph if dedicated well pumps will be used; otherwise delete.
All wells will be purged prior to sampling. If the well casing volume is known, a minimum of
three casing volumes of water will be purged using the dedicated well pump.
Include this paragraph if hand pumps, submersible pumps, bailers, or other sampling methods
will be used; otherwise delete.
All wells will be purged prior to sampling. If the well casing volume is known, a minimum of
three casing volumes of water will be purged using [specify sampling method]. When a
submersible pump is used for purging, clean flexible Teflon tubes will be used for groundwater
extraction. All tubes will be decontaminated before use in each well. Pumps will be placed 2 to
3 feet from the bottom of the well to permit reasonable draw down while preventing cascading
conditions.
The following paragraphs should be included in all sample plans.
Water will be collected into a measured bucket to record the purge volume. Casing volumes will
be calculated based on total well depth, standing water level, and casing diameter. One casing
volume will be calculated as:
V = πd
2
h / 77.01
where: V is the volume of one well casing of water (1ft
3
= 7.48 gallons);
d is the inner diameter of the well casing (in inches);
h is the total depth of water in the well (in feet).

SAP general 37 12/05/2011
It is most important to obtain a representative sample from the well. Stable water quality
parameter (temperature, pH and specific conductance) measurements indicate representative
sampling is obtainable. Water quality is considered stable if for three consecutive readings:
• temperature range is no more than +1°C;
• pH varies by no more than 0.2 pH units;
• specific conductance readings are within 10% of the average.
The water in which measurements were taken will not be used to fill sample bottles.
If the well casing volume is known, measurements will be taken before the start of purging, in
the middle of purging, and at the end of purging each casing volume. If the well casing volume
is NOT known, measurements will be taken every 2.5 minutes after flow starts. If water quality
parameters are not stable after 5 casing volumes or 30 minutes, purging will cease, which will be
noted in the logbook, and ground water samples will be taken. The depth to water, water quality
measurements and purge volumes will be entered in the logbook.
If a well dewaters during purging and three casing volumes are not purged, that well will be
allowed to recharge up to 80% of the static water column and dewatered once more. After water
levels have recharged to 80% of the static water column, groundwater samples will be collected.
6.5.2.3 Well Sampling
Describe the method that will be used to collect samples from wells. (This will probably be the
same method as was used to purge the wells.) Specify the sequence for sample collection (e.g.,
bottles for volatile analysis will be filled first, followed by semivolatiles, etc.). State whether
samples for metals analysis will be filtered or unfiltered. Include the specific conditions, such as
turbidity, that will require samples to be filtered. Alternatively, reference the appropriate
sections in the Ground Water SOP and state in which appendix the SOP is located.
The following paragraph should be included in all sample plans. Modify sample numbers and
analyses as necessary to suit the subject project.
At each sampling location, all bottles designated for a particular analysis (e.g., volatile organic
compounds) will be filled sequentially before bottles designated for the next analysis are filled
(e.g., semivolatile organic compounds). If a duplicate sample is to be collected at this location,
all bottles designated for a particular analysis for both sample designations will be filled

SAP general 38 12/05/2011
sequentially before bottles for another analysis are filled. In the filling sequence for duplicate
samples, bottles with the two different sample designations will alternate (e.g., volatile organic
compounds designation GW-2, volatile organic compounds designation GW-4 (duplicate of
GW-2), metals designation GW-2, and metals designation GW-4 (duplicate of GW-2).
Groundwater samples will be transferred directly into the appropriate sample containers with
preservative, if required, chilled if appropriate, and processed for shipment to the laboratory.
If samples are to be collected for volatiles analysis, include the following paragraph; otherwise
delete.
Samples for volatile organic compound analyses will be collected using a low flow sampling
device. A [specify type] pump will be used at a flow rate of ______. Vials for volatile organic
compound analysis will be filled first to minimize the effect of aeration on the water sample. See
Section 7.3 for preservation and shipping procedures.
If some samples for metals (or other) analysis are to be filtered, depending upon sample
turbidity, include the following paragraph; otherwise delete.
After well purging and prior to collecting groundwater samples for metals analyses, the turbidity
of the groundwater extracted from each well will be measured using a portable turbidity meter. A
small quantity of groundwater will be collected from the well, transferred to a disposable vial and
a turbidity measurement will be taken. The results of the turbidity measurement will be recorded
in the field logbook. The water used to measure turbidity will be discarded after use. If the
turbidity of the groundwater from a well is above 5 Nephelometric Turbidity Units (NTUs), both
a filtered and unfiltered sample will be collected. A 5-micron filter will be used to remove larger
particles that have been entrained in the water sample. A clean, unused filter will be used for each
filtered sample collected. Groundwater samples will be transferred from the filter directly into the
appropriate sample containers with a preservative and processed for shipment to the laboratory.
When transferring samples, care will be taken not to touch the filter to the sample container.
After the filtered sample has been collected, the Teflon tube and filter will be removed and an
unfiltered sample will be collected. A sample number appended with an “Fl” will represent a
sample filtered with a 5-micron filter. See Section 7.3 for preservation and shipping procedures.
If samples are to be filtered for metals (or other) analysis regardless of sample turbidity, include
the following paragraph; otherwise delete.

SAP general 39 12/05/2011
Samples designated for metals analysis will be filtered. A 5-micron filter will be used to remove
larger particles that have been entrained in the water sample. A clean, unused filter will be used
for each filtered sample collected. Groundwater samples will be transferred from the filter
directly into the appropriate sample containers to which preservative has been added and
processed for shipment to the laboratory. When transferring samples, care will be taken not to
touch the filter to the sample container. After the filtered sample has been collected, the Teflon
tube and filter will be removed and an unfiltered sample will be collected. A sample number
appended with an “Fl” will represent a sample filtered with a 5-micron filter. See Section 7.3 for
preservation and shipping procedures.
6.6 Other
Describe the collection of other media, if any.
6.7 Decontamination Procedures
Specify the decontamination procedures that will be followed if non-dedicated sampling
equipment is used. Alternatively, reference the appropriate sections in the organization’s
Decontamination SOP and state in which appendix the SOP is located.
The decontamination procedures that will be followed are in accordance with approved
procedures. Decontamination of sampling equipment must be conducted consistently as to
assure the quality of samples collected. All equipment that comes into contact with potentially
contaminated soil or water will be decontaminated. Disposable equipment intended for one-time
use will not be decontaminated, but will be packaged for appropriate disposal. Decontamination
will occur prior to and after each use of a piece of equipment. All sampling devices used,
including trowels and augers, will be steam-cleaned or decontaminated according to EPA Region
9 recommended procedures.
The following, to be carried out in sequence, is an EPA Region 9 recommended procedure for
the decontamination of sampling equipment
Use the following decontamination procedures; edit as necessary.
• Non-phosphate detergent and tap water wash, using a brush if necessary
• Tap-water rinse
• 0.1 N nitric acid rinse [For inorganic analyses, include an acid rinse; otherwise, delete.]

SAP general 40 12/05/2011
• Deionized/distilled water rinse
• Pesticide-grade solvent (reagent grade hexane) rinse in a decontamination bucket [For
organic analyses, include a solvent rinse; otherwise, delete.]
• Deionized/distilled water rinse (twice)
Equipment will be decontaminated in a predesignated area on pallets or plastic sheeting, and clean bulky
equipment will be stored on plastic sheeting in uncontaminated areas. Cleaned small equipment will be
stored in plastic bags. Materials to be stored more than a few hours will also be covered.
NOTE: If a different decontamination procedure is used; a rationale for using the different approach
should be provided.

SAP general 41 12/05/2011
Table 6-1: Field and Sampling Equipment
Description of Equipment
Material (if applicable)
Dedicated
(Yes/No)

SAP general 42 12/05/2011
Table 6-2: Field Equipment/Instrument Calibration, Maintenance, Testing, and Inspection
Analytical
Parameter
Field
Equipment/
Instrument
Calibration
Activity
Maintenance
& Testing/
Inspection
Activity
Frequency
Acceptance
Criteria
Corrective Action

SAP general 43 12/05/2011
7.0 SAMPLE CONTAINERS, PRESERVATION, PACKAGING AND SHIPPING
This section describes the types of containers to be used and the procedures for preserving,
packaging and shipping samples. Information concerning the number /type of sample
containers, volumes, and preservatives may have been presented in tabular form previously. The
organization responsible for adding preservatives should be named.
The number and type of sample containers, volumes, and preservatives are listed in [specify
table(s)]. The containers are pre-cleaned and will not be rinsed prior to sample collection.
Preservatives, if required, will be added by _______ [name of agency/organization doing the
sampling] to the containers prior to shipment of the samples to the laboratory.
7.1 Soil Samples
Include the following paragraphs, as appropriate; otherwise delete. Modify if necessary.
VOLATILE ORGANIC COMPOUNDS: Soil samples to be analyzed for volatile organic
compounds will be stored in their sealed _______ samplers for no more than two days prior to
analysis. Samples will be chilled to 4°C immediately upon collection.
Include the following sentences if samples will be frozen or preserved; otherwise delete.
Frozen Encore-sampler samples will be stored for no more than 4 days prior to analysis. If
samples are preserved by ejecting into either methanol or sodium bisulfate solution the holding
time is two weeks.
OTHER ORGANIC COMPOUNDS: Soil samples for ______________ [include all requested
analysis(ses)] will be homogenized and transferred from the sample-dedicated homogenization
pail into 8-ounze wide-mouth glass jars using a trowel. A separate container will be collected for
each laboratory. [Alternatively, samples will be retained in the brass sleeve in which collected
until sample preparation begins.] The samples will be chilled to 4°C immediately upon
collection.
METALS: Surface soil samples to be analyzed for metals will be homogenized and transferred
from the sample-dedicated homogenization pail into 8-oz, wide-mouth glass jars. A separate

SAP general 44 12/05/2011
container will be collected for each laboratory. Samples will not be chilled. Subsurface samples
will be retained in their original brass sleeves or other container unless transferred to bottles.
7.2 Sediment Samples
Include the following paragraphs, as appropriate; otherwise delete. Modify if necessary.
VOLATILE ORGANIC COMPOUNDS: Sediment samples to be analyzed for volatile organic
compounds will be stored in their sealed _________ samplers for no more than two days prior to
analysis. Samples will be chilled to 4°C immediately upon collection.
Include the following sentences if samples will be frozen or preserved; otherwise delete.
Frozen Encore-sampler samples will be stored for no more than 4 days prior to analysis. If
samples are preserved by ejecting into either methanol or sodium bisulfate solution the holding
time is two weeks.
OTHER ORGANIC COMPOUNDS: Soil samples for ______________ [include all requested
analysis(ses)] will be homogenized and transferred from the sample-dedicated homogenization
pail into 8-ounze wide-mouth glass jars using a trowel. A separate container will be collected for
each laboratory. The samples will be chilled to 4°C immediately upon collection.
METALS: Sediment samples, with rocks and debris removed, which are to be analyzed for
metals will be homogenized and transferred from the sample-dedicated homogenization pail into
8-oz, wide-mouth glass jars. A separate container will be collected for each laboratory. Samples
will not be chilled.
7.3 Water Samples
Include the following paragraphs, as appropriate; otherwise delete. Modify if necessary.
VOLATILE ORGANIC COMPOUNDS: Low concentration water samples to be analyzed for
volatile organic compounds will be collected in 40-ml glass vials. 1:1 hydrochloric acid (HCl)
will be added to the vial prior to sample collection. During purging, a test vial will be filled with
sample at each sample location and the pH will be measured using a pH meter or pH paper to
ensure that sufficient acid is present to result in a pH of less than 2. If the pH is greater than 2,

SAP general 45 12/05/2011
additional HCl will be added to the sample vials. Another vial will be pH tested to ensure the pH
is less than 2. The tested vial(s) will be discarded. The sample vials will be filled so that there is
no headspace. The vials will be inverted and checked for air bubbles to ensure zero headspace.
If a bubble appears, the vial will be discarded and a new sample will be collected. The samples
will be chilled to 4°C immediately upon collection. Three vials of each water sample are
required for each laboratory.
METALS: Water samples collected for metals analysis will be collected in 1-liter polyethylene
bottles. The samples will be preserved by adding nitric acid (HNO
3
) to the sample bottle. The
bottle will be capped and lightly shaken to mix in the acid. A small quantity of sample will be
poured into the bottle cap where the pH will be measured using pH paper. The pH must be < 2.
The sample in the cap will be discarded, and the pH of the sample will be adjusted further if
necessary. The samples will be chilled to 4°C immediately upon collection. One bottle of each
water sample is required for each laboratory.
GENERAL CHEMISTRY (WATER QUALITY) PARAMETERS: Water samples collected for
[specify the parameters requiring preservation] will be collected in [specify size of container]
polyethylene bottles. The [specify analysis] samples will be preserved by adding [describe
preservative appropriate to each sample type] to the sample bottle. The bottle will be capped
and lightly shaken to mix in the preservative. A small quantity of sample will be poured into the
bottle cap where the pH will be measured using pH paper. The pH must be within the
appropriate range. The sample in the cap will be discarded, and the pH of the sample will be
adjusted further if necessary. Samples will be chilled to 4°C immediately upon collection.
Samples from each location that require the same preservative will be placed in the same bottle,
if being analyzed by the same laboratory.
______________ [Include all requested analysis(es), e.g., Anions, Pesticides, Semivolatile
Organic Compounds. A separate paragraph should be included for each bottle type.] Low
concentration water samples to be analyzed for ______________ [specify analysis(ses)] will be
collected in ____________ [specify bottle type]. No preservative is required for these samples.
The samples will be chilled to 4°C immediately upon collection. Two bottles of each water
sample are required for each laboratory.
7.4 Other Samples

SAP general 46 12/05/2011
If samples of other media (e.g., soil gas) are to be collected, specify the analyses that will be
performed and the containers and preservatives required, if any.
7.5 Packaging and Shipping
The following provides a generic explanation and description of how to pack and ship samples.
It may be incorporated as is, if appropriate, or modified to meet any project-specific conditions.
All sample containers will be placed in a strong-outside shipping container (a steel-belted
cooler). The following outlines the packaging procedures that will be followed for low
concentration samples.
1. When ice is used, pack it in zip-locked, double plastic bags. Seal the drain plug of the
cooler with fiberglass tape to prevent melting ice from leaking out of the cooler.
2. The bottom of the cooler should be lined with bubble wrap to prevent breakage during
shipment.
3. Check screw caps for tightness and, if not full, mark the sample volume level of liquid
samples on the outside of the sample bottles with indelible ink.
4. Secure bottle/container tops with clear tape and custody seal all container tops.
5. Affix sample labels onto the containers with clear tape.
6. Wrap all glass sample containers in bubble wrap to prevent breakage.
7. Seal all sample containers in heavy duty plastic zip-lock bags. Write the sample numbers
on the outside of the plastic bags with indelible ink.
8. Place samples in a sturdy cooler(s) lined with a large plastic trash bag. Enclose the
appropriate COC(s) in a zip-lock plastic bag affixed to the underside of the cooler lid.
9. Fill empty space in the cooler with bubble wrap or Styrofoam peanuts to prevent
movement and breakage during shipment. Vermiculite should also be placed in the
cooler to absorb spills if they occur.
SAP general 47 12/05/2011
10. Ice used to cool samples will be double sealed in two zip lock plastic bags and placed on
top and around the samples to chill them to the correct temperature.
11. Each ice chest will be securely taped shut with fiberglass strapping tape, and custody
seals will be affixed to the front, right and back of each cooler.

SAP general 48 12/05/2011
8.0 DISPOSAL OF RESIDUAL MATERIALS
This section should describe the type(s) of investigation-derived wastes (IDW) that will be
generated during this sampling event. IDW may not be generated in all sampling events, in
which case this section would not apply. Use the language below or reference the appropriate
sections in a “Disposal of Residual Materials SOP” and state in which appendix the SOP is
located. Depending upon site-specific conditions and applicable federal, state, and local
regulations, other provisions for IDW disposal may be required. If any analyses of IDW are
required, these should be discussed. If IDW are to be placed in drums, labeling for the drums
should be discussed in this section.
In the process of collecting environmental samples, the sampling team will generate different
types of potentially contaminated IDW that include the following:
• Used personal protective equipment (PPE)
• Disposable sampling equipment
• Decontamination fluids
• Soil cuttings from soil borings [Include this bullet when sampling soils; otherwise
delete.]
• Purged groundwater and excess groundwater collected for sample
container filling [Include this bullet when sampling groundwater;
otherwise delete.]
The EPA's National Contingency Plan (NCP) requires that management of IDW generated
during sampling comply with all applicable or relevant and appropriate requirements (ARARs)
to the extent practicable. The sampling plan will follow the Office of Emergency and Remedial
Response (OERR) Directive 9345.3-02 (May 1991), which provides the guidance for the
management of IDW. In addition, other legal and practical considerations that may affect the
handling of IDW will be considered.
Listed below are the procedures that should be followed for handling the IDW. The procedures
have enough flexibility to allow the sampling team to use its professional judgment as to the
proper method for the disposal of each type of IDW generated at each sampling location.
The following bullet is generally appropriate for site or sampling areas with low levels of
contamination or for routine monitoring. If higher levels of contamination exist at the site or

SAP general 49 12/05/2011
sampling area, other disposal methods (such as the drumming of wastes) should be used to
dispose of used PPE and disposable sampling equipment.
• Used PPE and disposable equipment will be double bagged and placed in a municipal
refuse dumpster. These wastes are not considered hazardous and can be sent to a
municipal landfill. Any PPE and disposable equipment that is to be disposed of which
can still be reused will be rendered inoperable before disposal in the refuse dumpster.
Include this bullet if sampling for both metals and organics; otherwise delete.
• Decontamination fluids that will be generated in the sampling event will consist of dilute
nitric acid, pesticide-grade solvent, deionized water, residual contaminants, and water
with non-phosphate detergent. The volume and concentration of the decontamination
fluid will be sufficiently low to allow disposal at the site or sampling area. The water
(and water with detergent) will be poured onto the ground or into a storm drain.
Pesticide-grade solvents will be allowed to evaporate from the decontamination bucket.
The nitric acid will be diluted and/or neutralized with sodium hydroxide and tested with
pH paper before pouring onto the ground or into a storm drain.
Include this bullet if sampling for metals but not organics; otherwise delete.
• Decontamination fluids that will be generated in the sampling event will consist of nitric
acid, deionized water, residual contaminants, and water with non-phosphate detergent.
The volume and concentration of the decontamination fluid will be sufficiently low to
allow disposal at the site or sampling area. The water (and water with detergent) will be
poured onto the ground or into a storm drain. The nitric acid will be diluted and/or
neutralized with sodium hydroxide and tested with pH paper before pouring onto the
ground or into a storm drain.
Include this bullet if sampling for organics but not metals; otherwise delete.
• Decontamination fluids that will be generated in the sampling event will consist of
pesticide-grade solvent, deionized water, residual contaminants, and water with non-
phosphate detergent. The volume and concentration of the decontamination fluid will be
sufficiently low to allow disposal at the site or sampling area. The water (and water with
detergent) will be poured onto the ground or into a storm drain. Pesticide-grade solvents
will be allowed to evaporate from the decontamination bucket.

SAP general 50 12/05/2011
Include this bullet if sampling soils; otherwise delete.
• Soil cuttings generated during the subsurface sampling will be disposed of in an
appropriate manner.
Include this bullet if sampling groundwater; otherwise delete.
• Purged groundwater will be ______________
Depending upon the degree of groundwater contamination, site-specific conditions, and
applicable federal, state, and local regulations, disposal methods will vary. Disposal methods
can also vary for purge water from different wells sampled during the same sampling event.

SAP general 51 12/05/2011
9.0 SAMPLE DOCUMENTATION AND SHIPMENT
9.1 Field Notes
This section should discuss record keeping in the field. This may be through a combination of
logbooks, preprinted forms, photographs, or other documentation. Information to be maintained
is provided below.
9.1.1 Field Logbooks
Use field logbooks to document where, when, how, and from whom any vital project information
was obtained. Logbook entries should be complete and accurate enough to permit
reconstruction of field activities. Maintain a separate logbook for each sampling event or
project. Logbooks should have consecutively numbered pages. All entries should be legible,
written in black ink, and signed by the individual making the entries. Use factual, objective
language.
At a minimum, the following information will be recorded during the collection of each sample:
Edit this list as relevant.
Sample location and description
Site or sampling area sketch showing sample location and measured distances
Sampler's name(s)
Date and time of sample collection
Designation of sample as composite or grab
Type of sample (soil, sediment or water)
Type of sampling equipment used
Field instrument readings and calibration
Field observations and details related to analysis or integrity of samples (e.g., weather
conditions, noticeable odors, colors, etc.)
Preliminary sample descriptions (e.g., for soils: clay loam, very wet; for water: clear
water with strong ammonia-like odor)
Sample preservation
Lot numbers of the sample containers, sample identification numbers and any
explanatory codes, and chain-of-custody form numbers
Shipping arrangements (overnight air bill number)

SAP general 52 12/05/2011
Name(s) of recipient laboratory(ies)
In addition to the sampling information, the following specific information will also be recorded
in the field logbook for each day of sampling:
Edit this list as relevant.
Team members and their responsibilities
Time of arrival/entry on site and time of site departure
Other personnel on site
Summary of any meetings or discussions with tribal, contractor, or federal agency
personnel
Deviations from sampling plans, site safety plans, and QAPP procedures
Changes in personnel and responsibilities with reasons for the changes
Levels of safety protection
Calibration readings for any equipment used and equipment model and serial number
9.1.2 Photographs
If photographs will be taken, the following language may be used as is or modified as
appropriate.
Photographs will be taken at the sampling locations and at other areas of interest on the site or
sampling area. They will serve to verify information entered in the field logbook. For each
photograph taken, the following information will be written in the logbook or recorded in a
separate field photography log:
Time, date, location, and weather conditions
Description of the subject photographed
Name of person taking the photograph
9.2 Labeling
The following paragraph provides a generic explanation and description of the use of labels. It
may be incorporated as is, if appropriate, or modified to meet any project-specific conditions.

SAP general 53 12/05/2011
All samples collected will be labeled in a clear and precise way for proper identification in the
field and for tracking in the laboratory. A copy of the sample label is included in Appendix __.
The samples will have pre-assigned, identifiable, and unique numbers. At a minimum, the
sample labels will contain the following information: station location, date of collection,
analytical parameter(s), and method of preservation. Every sample, including samples collected
from a single location but going to separate laboratories, will be assigned a unique sample
number.
9.3 Sample Chain-Of-Custody Forms and Custody Seals
The following paragraphs provide a generic explanation and description of the use of chain-of-
custody forms and custody seals. They may be incorporated as is, if they are appropriate, or
modified to meet any project-specific conditions.
All sample shipments for analyses will be accompanied by a chain-of-custody record. A copy of
the form is found in Appendix __. Form(s) will be completed and sent with the samples for each
laboratory and each shipment (i.e., each day). If multiple coolers are sent to a single laboratory
on a single day, form(s) will be completed and sent with the samples for each cooler.
The chain-of-custody form will identify the contents of each shipment and maintain the custodial
integrity of the samples. Generally, a sample is considered to be in someone’s custody if it is
either in someone’s physical possession, in someone's view, locked up, or kept in a secured area
that is restricted to authorized personnel. Until the samples are shipped, the custody of the
samples will be the responsibility of ____________ [name of agency/ organization conducting
sampling]. The sampling team leader or designee will sign the chain-of-custody form in the
“relinquished by” box and note date, time, and air bill number.
A self-adhesive custody seal will be placed across the lid of each sample. A copy of the seal is
found in Appendix ____. For VOC samples, the seal will be wrapped around the cap. The
shipping containers in which samples are stored (usually a sturdy picnic cooler or ice chest) will
be sealed with self-adhesive custody seals any time they are not in someone's possession or view
before shipping. All custody seals will be signed and dated.

SAP general 54 12/05/2011
10.0 QUALITY CONTROL
The following sections should discuss the quality control samples that are being collected to
support the sampling activity. This includes field QC samples, confirmation samples,
background samples, laboratory QC samples, and split samples. Wherever possible, the
locations at which the samples will be collected should be identified and a rationale provided for
the choice of location. Frequency of collection should be discussed.
10.1 Field Quality Control Samples
Field quality control samples are intended to help evaluate conditions resulting from field
activities and are intended to accomplish two primary goals, assessment of field contamination
and assessment of sampling variability. The former looks for substances introduced in the field
due to environmental or sampling equipment and are assessed using blanks of different types.
The latter includes variability due to sampling technique and instrument performance as well as
variability possibly caused by the heterogeneity of the matrix being sampled and is assessed
using replicate sample collection. The following subsections cover field QC.
10.1.1 Assessment of Field Contamination (Blanks)
Field contamination is usually assessed through the collection of different types of blanks.
Equipment blanks are obtained by passing distilled or deionized water, as appropriate, over or
through the decontaminated equipment used for sampling. They provide the best overall means
of assessing contamination arising from the equipment, ambient conditions, sample containers,
transit, and the laboratory. Field blanks are sample containers filled in the field. They help
assess contamination from ambient conditions, sample containers, transit, and the laboratory.
Trip blanks are prepared by the laboratory and shipped to and from the field. They help assess
contamination from shipping and the laboratory and are for volatile organic compounds only.
Region 9 recommends that equipment blanks be collected, where appropriate (e.g., where
neither disposable nor dedicated equipment is used). Field blanks are next in priority, followed
by trip blanks. Only one type of blank must be collected per event, not all three.
A maximum of one blank sample per matrix per day should be collected, but at a rate not to
exceed one blank per 10 samples. The 1:10 ratio overrides the one per day requirement. If
equipment rinsate blanks are collected, field blanks and trip blanks are not required under
normal circumstances.

SAP general 55 12/05/2011
10.1.1.1 Equipment Blanks
In general, equipment (rinsate) blanks should be collected when reusable, non-disposable
sampling equipment (e.g., trowels, hand augers, and non-dedicated groundwater sampling
pumps) are being used for the sampling event. Equipment blanks can be collected for soil,
sediment, surface water and ground water samples. A minimum of one equipment blank is
prepared each day for each matrix when equipment is decontaminated in the field. These blanks
are submitted “blind” to the laboratory, packaged like other samples and assigned its own
unique identification number. Note that for samples which may contain VOCs, water for blanks
should be purged prior to use to ensure that it is organic free. High Performance Liquid
Chromatography (HPLC) water, which is often used for equipment and field blanks, can contain
VOCs if it is not purged.
Include this paragraph if blanks will be analyzed for both metals and organic compounds;
otherwise delete.
Equipment rinsate blanks will be collected to evaluate field sampling and decontamination
procedures by pouring High Performance Liquid Chromatography (HPLC) organic-free (for
organics) or deionized water (for inorganics) over the decontaminated sampling equipment. One
equipment rinsate blank will be collected per matrix each day that sampling equipment is
decontaminated in the field. Equipment rinsate blanks will be obtained by passing water through
or over the decontaminated sampling devices used that day. The rinsate blanks that are collected
will be analyzed for ________. [Include names of target analytes, e.g., metals, total petroleum
hydrocarbons, volatile organic compounds, etc.]
Include this paragraph if blanks will be analyzed only for organic compounds; otherwise delete.
Equipment rinsate blanks will be collected to evaluate field sampling and decontamination
procedures by pouring High Performance Liquid Chromatography (HPLC) organic-free water
over the decontaminated sampling equipment. One equipment rinsate blank will be collected per
matrix each day that sampling equipment is decontaminated in the field. Equipment rinsate
blanks will be obtained by passing water through or over the decontaminated sampling devices
used that day. The rinsate blanks that are collected will be analyzed for _________. [Include
names of target analytes, e.g., volatile organic compounds, total petroleum hydrocarbons, etc.]
Include this paragraph if blanks will be analyzed only for metals; otherwise delete.

SAP general 56 12/05/2011
Equipment rinsate blanks will be collected to evaluate field sampling and decontamination
procedures by pouring deionized water over the decontaminated sampling equipment. One
equipment rinsate blank will be collected per matrix each day that sampling equipment is
decontaminated in the field. Equipment rinsate blanks will be obtained by passing deionized
water through or over the decontaminated sampling devices used that day. The rinsate blanks
that are collected will be analyzed for metals.
Always include this paragraph.
The equipment rinsate blanks will be preserved, packaged, and sealed in the manner described
for the environmental samples. A separate sample number and station number will be assigned
to each sample, and it will be submitted blind to the laboratory.
10.1.1.2 Field Blanks
Field blanks are collected when dedicated equipment is used and decontamination is not
necessary. A minimum of one field blank is prepared each day sampling occurs in the field, but
equipment is not decontaminated. These blanks are submitted “blind” to the laboratory,
packaged like other samples and each with its own unique identification number. Note that for
samples which may contain VOCs, water for blanks should be purged prior to use to ensure that
it is organic free. HPLC water, which is often used for equipment and field blanks, can contain
VOCs if it is not purged.
Include this paragraph if blanks will be analyzed for both metals and organic compounds;
otherwise delete.
Field blanks will be collected to evaluate whether contaminants have been introduced into the
samples during the sampling due to ambient conditions or from sample containers. Field blank
samples will be obtained by pouring High Performance Liquid Chromatography (HPLC)
organic-free water (for organics) and/or deionized water (for inorganics) into a sampling
container at the sampling point. The field blanks that are collected will be analyzed for
_________. [Include names of target analytes, e.g., metals, volatile organic compounds, etc.]
Include this paragraph if blanks will be analyzed only for organic compounds; otherwise delete.
Field blanks will be collected to evaluate whether contaminants have been introduced into the
samples during the sampling due to ambient conditions or from sample containers. Field blank

SAP general 57 12/05/2011
samples will be obtained by pouring High Performance Liquid Chromatography (HPLC)
organic-free water into a sampling container at the sampling point. The field blanks that are
collected will be analyzed for _________. [Include names of target analytes, e.g., volatile
organic compounds, total petroleum hydrocarbons, etc.]
Include this paragraph if blanks will be analyzed only for metals; otherwise delete.
Field blanks will be collected to evaluate whether contaminants have been introduced into the
samples during the sampling due to contamination from sample containers. Field blank samples
will be obtained by pouring deionized water into a sampling container at the sampling point.
The field blanks that are collected will be analyzed for metals.
Always include this paragraph.
The field blanks will be preserved, packaged, and sealed in the manner described for the
environmental samples. A separate sample number and station number will be assigned to each
sample, and it will be submitted blind to the laboratory.
10.1.1.3 Trip Blanks
Trip blanks are required only if no other type of blank will be collected. Trip blanks are only
relevant to volatile organic compound (VOC) sampling efforts. If trip blanks are required, one
is submitted to the laboratory for analysis with every shipment of samples for VOC analysis.
These blanks are submitted “blind” to the laboratory, packaged like other samples and each is
assigned its own unique identification number. Note that for samples which may contain VOCs,
water for blanks should be purged prior to use to ensure that it is organic free. HPLC water,
which is often used for trip blanks, can contain VOCs if it is not purged.
Trip blanks will be prepared to evaluate if the shipping and handling procedures are introducing
contaminants into the samples, and if cross contamination in the form of VOC migration has
occurred between the collected samples. A minimum of one trip blank will be submitted to the
laboratory for analysis with every shipment of samples for VOC analysis. Trip blanks are 40-mL
vials that have been filled with HPLC-grade water that has been purged so it is VOC free and
shipped with the empty sampling containers to the site or sampling area prior to sampling. The
sealed trip blanks are not opened in the field and are shipped to the laboratory in the same cooler
with the samples collected for volatile analyses. The trip blanks will be preserved, packaged,
and sealed in the manner described for the environmental samples. A separate sample number

SAP general 58 12/05/2011
and station number will be assigned to each trip sample and it will be submitted blind to the
laboratory.
10.1.1.4 Temperature Blanks
Include this paragraph with all plans.
For each cooler that is shipped or transported to an analytical laboratory a 40-mL VOA vial will
be included that is marked “temperature blank.” This blank will be used by the sample custodian
to check the temperature of samples upon receipt.
10.1.2 Assessment of Field Variability (Field Duplicate or Co-located Samples
Duplicate samples are collected simultaneously with a standard sample from the same source
under identical conditions but are placed into separate sample containers. Field duplicates will
consist of a homogenized sample divided in two or else a co-located sample. Each duplicate
portion should be assigned its own sample number so that it will be blind to the laboratory. A
duplicate sample is treated independently of its counterpart to enable assessment of laboratory
performance through comparison of the results. At least 10% of samples collected per event
should be field duplicates. At least one duplicate should be collected for each sample matrix, but
collection can be stretched out over more than one day (e.g., if it takes more than one day to
reach 10 samples). Every group of analytes for which a standard sample is analyzed will also
include the analyses of one or more duplicate samples. Duplicate samples should be collected
from areas of known or suspected contamination. Since the objective is to assess variability due
to sampling technique and possible sample heterogeneity, source variability is a good reason to
collect co-located samples, not to avoid their collection.
Duplicate soils samples will be collected at sample locations _______________. [Identify soil
sample locations from which duplicate or collocated samples will be collected.] Duplicate
samples will be collected from these locations because ________________. [Include a rationale
for collecting duplicate samples from these locations; e.g., “samples from these locations are
suspected to exhibit moderate concentrations of contaminants” or “previous sampling events
have detected moderate levels of contamination at the site or sampling area at these locations.”]
Include this paragraph if collecting soil samples and analyzing for compounds other than
volatiles; otherwise delete.

SAP general 59 12/05/2011
Soil samples to be analyzed for __________________ [list all analytical methods for this
sampling event except for volatiles] will be homogenized with a trowel in a sample-dedicated
one gallon disposable pail. Homogenized material from the bucket will then be transferred to the
appropriate wide-mouth glass jars for both the regular and duplicate samples. All jars designated
for a particular analysis (e.g., semivolatile organic compounds) will be filled sequentially before
jars designated for another analysis are filled (e.g., metals).
Include this paragraph if collecting soil samples and analyzing for volatiles; otherwise delete.
Soil samples for volatile organic compound analyses will not be homogenized. Equivalent
samples from a co-located location will be collected identically to the original samples, assigned
unique sample numbers and sent blind to the laboratory.
Include these paragraphs if collecting sediment samples. If volatile organic compound analysis
will be performed on sediment samples, modify the above paragraph for soil sample volatile
analyses by changing “soil” to “sediment.”
Duplicate sediment samples will be collected at sample locations _______________. [Identify
sediment sample locations from which duplicate or co-located samples for duplicate analysis
will be obtained.] Duplicate samples will be collected from these locations because
________________. [Include a rationale for collecting duplicate samples from these locations.]
Sediment samples will be homogenized with a trowel in a sample-dedicated 1-gallon disposable
pail. Homogenized material from the bucket will then be transferred to the appropriate wide-
mouth glass jars for both the regular and duplicate samples. All jars designated for a particular
analysis (e.g., semivolatile organic compounds) will be filled sequentially before jars designated
for another analysis are filled (e.g., metals).
Include this paragraph if collecting water samples; otherwise delete.
Duplicate water samples will be collected for water sample numbers _____________ [water
sample numbers which will be split for duplicate analysis]. Duplicate samples will be collected
from these locations because ________________. [Include a rationale for collecting duplicate
samples from these locations.]
When collecting duplicate water samples, bottles with the two different sample identification
numbers will alternate in the filling sequence (e.g., a typical filling sequence might be, VOCs

SAP general 60 12/05/2011
designation GW-2, VOCs designation GW-4 (duplicate of GW-2); metals, designation GW-2,
metals, designation GW-4, (duplicate of GW-2) etc.). Note that bottles for one type of analysis
will be filled before bottles for the next analysis are filled. Volatiles will always be filled first.
Always include this paragraph.
Duplicate samples will be preserved, packaged, and sealed in the same manner as other samples
of the same matrix. A separate sample number and station number will be assigned to each
duplicate, and it will be submitted blind to the laboratory.
10.2 Background Samples
Background samples are collected in situations where the possibility exists that there are native
or ambient levels of one or more target analytes present or where one aim of the sampling event
is to differentiate between on-site and off-site contributions to contamination. One or more
locations are chosen which should be free of contamination from the site or sampling location
itself, but have similar geology, hydrogeology, or other characteristics to the proposed sampling
locations that may have been impacted by site activities. For example, an area adjacent to but
removed from the site, upstream from the sampling points, or up gradient or cross gradient from
the groundwater under the site. Not all sampling events require background samples.
Specify the sample locations that have been designated as background. Include a rationale for
collecting background samples from these locations and describe or reference the sampling and
analytical procedures which will be followed to collect these samples.
10.3 Field Screening, Confirmation and Split Samples
For projects where field screening methods are used (typically defined as testing using field test
kits, immunoassay kits, or soil gas measurements or equivalent, but not usually defined as the
use of a mobile laboratory which generates data equivalent to a fixed laboratory), two aspects of
the tests should be described. First, the QC which will be run in conjunction with the field
screening method itself, and, second, any fixed laboratory confirmation tests which will be
conducted. QC acceptance criteria for these tests should be defined in these sections rather than
in the DQO section.
10.3.1 Field Screening Samples

SAP general 61 12/05/2011
For projects where field screening methods are used, describe the QC samples which will be run
in the field to ensure that the screening method is working properly. This usually consists of a
combination of field duplicates and background samples. The discussion should specify
acceptance criteria and corrective action to be taken if results are not within defined limits.
Discuss confirmation tests below.
10.3.2 Confirmation Samples
If the planned sampling event includes a combination of field screening and fixed laboratory
confirmation, this section should describe the frequency with which the confirmation samples
will be collected and the criteria which will be used to select confirmation locations. These will
both be dependent on the use of the data in decision making. It is recommended that the
selection process be at a minimum of 10% and that selection criteria include checks for both
false positives (i.e., the field detections are invalid or the concentrations are not accurate) and
false negatives (i.e., the analyte was not detected in the field). Because many field screening
techniques are less sensitive than laboratory methods, false negative screening is especially
important unless the field method is below the action level for any decision making. It is
recommended that some “hits” be chosen and that other locations be chosen randomly.
Describe confirmation sampling. Discuss the frequency with which samples will be confirmed
and how location will be chosen. Define acceptance criteria for the confirmation results and
corrective actions to be taken if samples are not confirmed.
10.3.3 Split Samples
Split Samples are defined differently by different organizations, but for the purpose of this
guidance, Region 9’s QA Office considers split samples as ones that are divided among two or
more laboratories for the purpose of providing an inter-laboratory or inter-organization
comparison. Usually one organization (for example, a responsible party) collects the samples
and provides sufficient material to the other organization (for example, EPA) to enable it to
perform independent analyses. It is expected that the sampling party will have prepared a
sampling plan which the QA Office has reviewed and approved that describes the sampling
locations and a rationale for their choice, sampling methods, and analyses.
Describe the purpose of the split sampling. Include references to the approved sampling plan
prepared by the party collecting the samples. Provide a rationale for the sample locations at
which split samples will be obtained and how these locations are representative of the sampling

SAP general 62 12/05/2011
event as a whole. Describe how results are to be compared and define criteria by which
agreement will be measured. Discuss corrective action to be taken if results are found to not be
in agreement.
10.4 Laboratory Quality Control Samples
Laboratory quality control (QC) samples are analyzed as part of standard laboratory practice.
The laboratory monitors the precision and accuracy of the results of its analytical procedures
through analysis of QC samples. Typically, laboratory QC samples consist of matrix
spike/matrix spike duplicate (MS/MSD) samples for organic analyses, and matrix spike and
duplicate samples (MS/DS) for inorganic analyses. The term “matrix” refers to use of the actual
media collected in the field (e.g., routine soil and water samples).
Laboratory QC samples are collected in the field, but prepared by the laboratory. They are not
a separate sample, but an aliquot (subset) of an existing field sample. Do not include laboratory
QC checks, such as calibration standards or surrogates. These were discussed in previous
sections and should be included in MQO tables or the laboratory QA Manual/SOPs.
Include the following language if soil samples are to be collected for other than volatiles;
otherwise delete.
A routinely collected soil sample (a full 8-oz sample jar or two 120-mL sample vials) contains
sufficient volume for both routine sample analysis and additional laboratory QC analyses.
Therefore, a separate soil sample for laboratory QC purposes will not be collected.
Include the following language if soil samples are to be collected for volatiles; otherwise delete.
Soil samples for volatile organic compound analyses for laboratory QC purposes will be
obtained by collecting double the number of equivalent Encore samples from a co-located
location in the same way as the original samples.
Include the following language if water samples are to be collected; otherwise delete.
For water samples, double volumes of samples are supplied to the laboratory for its use for QC
purposes. Two sets of water sample containers are filled and all containers are labeled with a
single sample number. [For volatile samples, this would result in 6 vials being collected instead
of 3, for pesticides and semivolatile samples this would be 4 liters instead of 2, etc.]

SAP general 63 12/05/2011
The laboratory should be alerted as to which sample is to be used for QC analysis by a notation
on the sample container label and the chain-of-custody record or packing list.
At a minimum, one laboratory QC sample is required per 14 days or one per 20 samples
(including blanks and duplicates), whichever is greater. If the sample event lasts longer than 14
days or involves collection of more than 20 samples per matrix, additional QC samples will be
designated.
For this sampling event, samples collected at the following locations will be the designated
laboratory QC samples:
If a matrix is not being sampled, delete the reference to that matrix.
For soil, samples ____________ [List soil sample locations and numbers designated for
QA/QC.]
For sediment, samples ____________ [List sediment sample locations and numbers
designated for QA/QC.]
For water, samples ____________ [List water sample locations and numbers designated
for QA/QC.]
Add a paragraph explaining why these sample locations were chosen for QA/QC samples.
QA/QC samples should be samples expected to contain moderate levels of contamination. A
rationale should justify the selection of QA/QC samples based on previously-detected
contamination at the site or sampling area, historic site or sampling area operations, expected
contaminant deposition/migration, etc.

SAP general 64 12/05/2011
11.0 FIELD VARIANCES
It is not uncommon to find that, on the actual sampling date, conditions are different from
expectations such that changes must be made to the SAP once the samplers are in the field. The
following paragraph provides a means for documenting those deviations, or variances. Adopt
the paragraph as is, or modify it to project-specific conditions.
As conditions in the field may vary, it may become necessary to implement minor modifications
to sampling as presented in this plan. When appropriate, the QA Office will be notified and a
verbal approval will be obtained before implementing the changes. Modifications to the
approved plan will be documented in the sampling project report.

SAP general 65 12/05/2011
12.0 FIELD HEALTH AND SAFETY PROCEDURES
Describe any agency-, program- or project-specific health and safety procedures that must be
followed in the field, including safety equipment and clothing that may be required, explanation
of potential hazards that may be encountered, and location and route to the nearest hospital or
medical treatment facility. A copy of the organization health and safety plan may be included in
the Appendix and referenced in this section.

SAP general 66 12/05/2011
ATTACHMENT 1
EXAMPLE FORMS
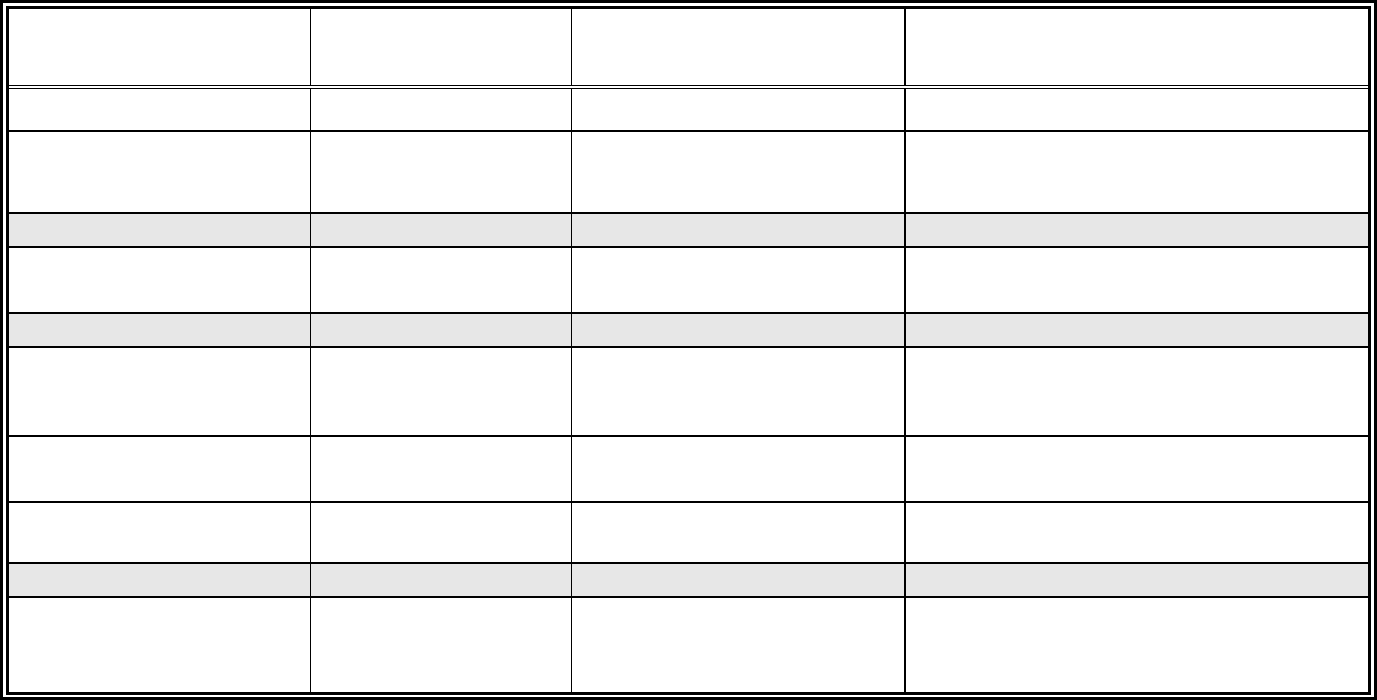
SAP general 67 12/05/2011
EXAMPLE
Table 1-1: Key Project Personnel Contact Information and Responsibilities
Title
Name
Phone Number
Email Address
Responsibilities
EPA Project Manager
EPA Quality Assurance
Officer (QAO)
Eugenia McNaughton,
Ph.D.
(415) 972-3411
Provide technical assistance
Approve sampling plans
Grantee Project Manager
Contractor Project
Manager (include
Company Name)
Contractor QAO
Contractor Field Team
Leader
Laboratory Quality
Assurance Officer (include
Laboratory Name)

SAP general 68 12/05/2011
EXAMPLE
Table 2-1: Contaminants of Concern – Previous Investigations
Matrix = Soil
Analytical Parameter
(Contaminants of Concern)
Date of sampling
Sampling contractor
Laboratory
Analytical Results
(units)
Regulatory Limit
(specify)
Benzene
1/1/01
ABC Co.
200 ug/KG
50 ug/Kg
1
ug/Kg = micrograms per kilogram
1
DTSC = Calif. Department of Toxic Substances Control

SAP general 69 12/05/2011
EXAMPLE
Table 3-1: Contaminants of Concern, Laboratory and Action Levels
Matrix = Soil
Analytical Parameter
(Contaminants of Concern)
Laboratory
Reporting or
Quantitation
Limits
Action Levels
EPA Residential
PRGs
DTSC Residential
CHHSLs
RWQCB
Residential ESLs
Volatile Organic Compounds by Method 8260 (ug/Kg)
Benzene
10
640
NA
440
Tetrachloroethylene (PCE)
10
480
NA
87
Toluene
10
520000
NA
3300
Metals by Method 6010/7470 (mg/Kg)
Arsenic
1
0.07
0.07
Background
Chromium
2
210
0
1000
Lead
2
150
150
150
EPA = US Environmental Protection Agency
DTSC = Calif. Department of Toxic Substances Control
RWQCB = Regional Water Quality Control Board
PRGs = Preliminary Remediation Goal (2004)
CHHSLs = California Human Health Screening Levels
ESLs = Environmental Screening Levels
NA = Not available or Not applicable
ug/Kg = micrograms per kilogram
mg/Kg = milligrams per kilogram

SAP general 70 12/05/2011
EXAMPLE
Table 4-1: Sampling Design and Rationale
Matrix = Soil
Sampling
Location/ID
Number
Depth
(ft)
Analytical
Parameter
Rationale *
SB1
0-1.5
2-4, 6-8
TPH-g/d, metals
TPH-g/d, VOA &
metals
Assess environmental conditions at the former UST
and former fuel pump island locations. Volatiles will
not be collected from the shallow soil due to probable
weathering effects.
SB2
0-1.5
2-4, 6-8
TPH-g/d, metals
TPH-g/d, VOA &
metals
Assess the potential presence of contaminants in
undocumented fill materials at the Site. V
olatiles will
not be collected from the shallow soil due to probable
weathering effects.
* Include rationale for location, depth and analysis.
TPH –g/d = total petroleum hydrocarbons as gasoline and diesel
VOA = volatile organic analyses

SAP general 71 12/05/2011
EXAMPLE
Table 4-2: Sampling Design and Rationale
Matrix = Groundwater
Sampling
Location/ID
Number
Analytical
Parameter
Rationale *
SB1
TPH-g/d, VOA,
metals
Assess the potential migration of contaminants to the
groundwater at the former UST and former fuel pump island
locations.
SB2
TPH-g/d, VOA,
metals
Assess the potential migration of contaminants to the
groundwater from the fill materials located on the Site.
* Include rationale for location and analysis.
TPH –g/d = total petroleum hydrocarbons as gasoline and diesel
VOA = volatile organic analyses

SAP general 72 12/05/2011
EXAMPLE
Table 5-1: Analytical Services
Matrix = Soil
Sample
Number
Sample
Location
Depth
(ft)
Special
Designation
Analytical Methods
SW846
Method
8015B
(TPH as
gasoline)
SW846
Method
8015B
(TPH as
diesel)
SW846
Method
8260B
(volatiles)
SW846
Method
6010/7470
(metals)
SB-01-05
SB1
0-1.5
X
X
X
SB-01-24
SB1
2-4
MS/MSD
X
X
X
X
SB-01-68
SB1
6-8
X
X
X
X
SB-02-05
SB2
0-1.5
X
X
X
SB-02-24
SB2
2-4
X
X
X
X
SB-02-68
SB2
6-8
X
X
X
X
SB-01-10
SB2
6-8
Duplicate of
SB-02-68
X
X
X
X
Total number of Soil Samples, excluding QC:
6
6
4
6
Total number of Soil Samples, including QC:
7
7
5
7
TPH = total petroleum hydrocarbons
MS/MSD = matrix spike/ matrix spike duplicate
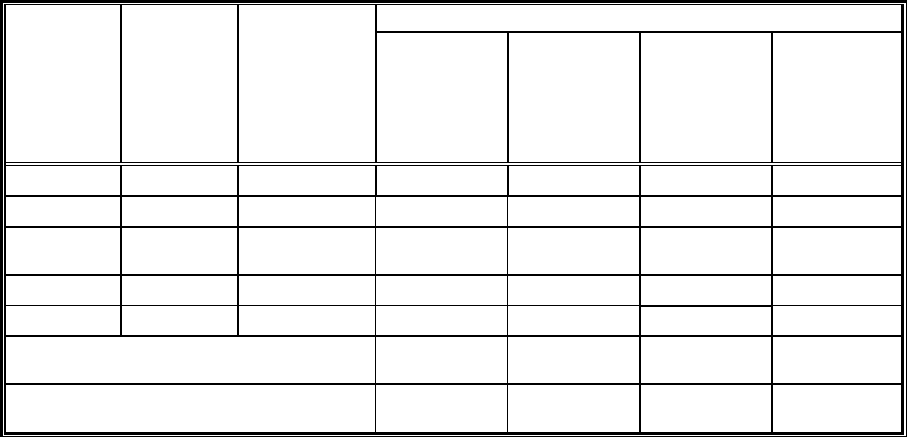
SAP general 73 12/05/2011
EXAMPLE
Table 5-2: Analytical Services
Matrix = Groundwater
Sample
Number
Sample
Location
Special
Designation
Analytical Methods
SW846
Method
8015B
(TPH as
gasoline)
SW846
Method
8015B
(TPH as
diesel)
SW846
Method
8260B
(volatiles)
SW846
Method
6010/7470
(metals)
SB-01
SB1
MS/MSD
X
X
X
X
SB-02
SB2
X
X
X
X
SB-03
SB2
Duplicate of
SB-02-68
X
X
X
X
Total number of Soil Samples, excluding
QC:
2
2
2
2
Total number of Soil Samples, including
QC:
3
3
3
3
TPH = total petroleum hydrocarbons
MS/MSD = matrix spike/ matrix spike duplicate

SAP general 74 12/05/2011
EXAMPLE
Table 5-3: Analytical Method, Containers, Preservation,
and Holding Times Requirements
Matrix = Soil
Analytical
Parameter
and/or Field
Measurements
Analytical
Method Number
Containers
(number, type,
size/volume)
Preservation
Requirements
(chemical,
temperature,
light protection)
Maximum
Holding Times
Volatiles
SW-846 Method
8260B
Two EnCore®
Samplers
Chill with ice to 4
o
C
48 hours
Metals
SW-846 Method
6010/7470
4 oz glass jar
Chill with ice to 4
o
C
<180 days/<28 days
for Hg
Table 5-4: Analytical Method, Containers, Preservation,
and Holding Times Requirements
Matrix = Groundwater
Analytical
Parameter
and/or Field
Measurements
Analytical
Method Number
Containers
(number, type,
size/volume)
Preservation
Requirements
(chemical,
temperature,
light protection)
Maximum
Holding Times
Volatiles
SW-846 Method
8260B
3 x 40-ml VOA
Chill with ice to 4
o
C
pH<2 with HCl
14 days
Metals
SW-846 Method
6010/7470
1 L HDPE
Chill with ice to 4
o
C
pH<2 with HNO
3
6 months
VOA = volatile organic analysis
HDPE = high density polyethylene
Hg = mercury
HCl = hydrochloric acid
HNO
3
= nitric acid

SAP general 75 12/05/2011
EXAMPLE
Table 6-1: Field and Sampling Equipment
Description of Equipment
Material (if applicable)
Dedicated
(Yes/No)
Sampling sleeves
Acetate or equivalent
Yes
Hand auger
Hardened steel
No
EnCore® samplers
Plastic
Yes
Sampling trowel
Plastic or stainless steel
Yes
Bailer
Plastic or stainless steel
Yes
Conductivity meter
NA
No
Peristaltic pump with dedicated
tubing
Tygon or HDPE tubing
No
NA = not applicable
HDPE = high density polyethylene

SAP general 76 12/05/2011
EXAMPLE
Table 6-2: Field Equipment/Instrument Calibration, Maintenance, Testing, and Inspection
Analytical
Parameter
Field
Equipment/
Instrument
Calibration
Activity
Maintenance
& Testing/
Inspection
Activity
Frequency
Acceptance
Criteria
Corrective Action
Temperature
(sensor)
Multimeter
Manufacturer X,
Model Y
Annual check of
endpoints of desired
temperature range (0
o
C
to 40
o
C) versus NIST
thermometer
See
manufacturer’s
manual
Annually
±0.2
o
C of true value at both
endpoints (i.e., manufacturer’s
listed accuracy for the sensor)
Remove from use if
doesn’t pass calibration
criteria
pH (electrode)
Multimeter
Manufacturer X,
Model Y
Initial: two-point
calibration bracketing
expected range (using
7.0 and either 4.0 or
10.0 pH buffer,
depending on field
conditions); followed
by one-point check
with 7.0 pH buffer
Post: single-point
check with 7.0 pH
buffer
See
manufacturer’s
manual
Initial:
beginning of
each day
Post: end of
each day
Initial: two-point calibration
done electronically; one-point
check (using 7.0 pH buffer) ±0.1
pH unit of true value
Post: ) ±0.5 pH unit of true
value with both 7.0 pH and other
“bracketing” buffer (and either
4.0 or 10.0 pH)
Recalibrate
Qualify data
