
Drinking Water Treatment Plant Residuals
Management
Technical Report
Summary of Residuals Generation, Treatment, and
Disposal at Large Community Water Systems
September 2011
EPA 820-R-11-003
United States
Environmental Protection
Agency

Drinking Water Industry Report
DISCLAIMER
This report on the drinking water treatment industry does not set forth any regulatory
requirements under the Clean Water Act. It is intended solely as a presentation of information of
which the U.S. Environmental Protection Agency (EPA) is currently aware concerning the
generation, treatment, and disposal of wastewater and solid residuals at water treatment plants
(WTPs). Thus, it does not impose any requirements on any party, including EPA, states,
permitting authorities, publicly-owned treatment works (POTWs), or the regulated community.
This report was prepared using information from the following sources: review of selected
literature, reports, and other materials; meetings with several interested parties; site visits at
WTPs; an industry survey; and other information solicited from stakeholders.
References made in this report to any specific method, product or process, vendor, or corporation
do not constitute or imply an endorsement, recommendation, or warranty by the U.S. EPA. EPA
does not assume any legal liability or responsibility for any third party’s use of, or the results of
such use of, any information discussed in this report, or represents that its use by such a third
party would not infringe on privately owned rights.
i

Drinking Water Industry Report Contents
CONTENTS
Page
SECTION 1 INTRODUCTION ..................................................................................................... 1-1
SECTION 2 DATA SOURCES ..................................................................................................... 2-1
2.1 Summary of EPA’s Water Treatment Plant Site Visits ........................... 2-1
2.2 EPA DWT Industry Questionnaire .......................................................... 2-2
2.2.1 Overview of Industry Questionnaire ............................................ 2-2
2.2.2 Description of Questionnaire ....................................................... 2-6
2.2.3 Development of the Survey Mailing List..................................... 2-7
2.2.4 Sample Selection .......................................................................... 2-8
2.2.5 Survey Response .......................................................................... 2-8
2.2.6 Protection of Confidential Business Information ........................ 2-9
2.3 EPA’s Ground Water and Drinking Water Data .................................... 2-10
2.3.1 Safe Drinking Water Information System ................................. 2-10
2.3.2 2000 Community Water System Survey.................................... 2-12
2.3.3 Information Collection Rule ...................................................... 2-13
2.3.4 Other Ground Water and Drinking Water Data ......................... 2-14
2.4 Other Information Collection Activities ................................................ 2-15
2.4.1 Literature Search ........................................................................ 2-15
2.4.2 Current NPDES Permits ............................................................ 2-16
2.4.3 NPDES Discharge Monitoring Reports (DMRs) ....................... 2-17
2.4.4 Other EPA Data ......................................................................... 2-18
2.4.5 Industry Data .............................................................................. 2-19
2.4.6 American Water Works Association (AWWA) Surveys and
Reports ....................................................................................... 2-20
2.5 Stakeholder Meetings............................................................................. 2-22
2.6 Drinking Water Treatment Technology Review.................................... 2-23
2.7 References .............................................................................................. 2-24
SECTION 3 INDUSTRY PROFILE ............................................................................................... 3-1
3.1 Overview of DWT Industry ..................................................................... 3-1
3.1.1 Types of Drinking Water Systems ............................................... 3-3
3.1.2 How EPA Classifies Drinking Water Systems ............................ 3-3
3.2 Summary of Questionnaire Responses .................................................... 3-4
3.2.1 System and WTP Classification................................................... 3-4
3.2.2 WTP Characteristics (Summary of Responses to Technical
Questions) .................................................................................... 3-6
3.3 Drinking Water Industry Economic Overview ...................................... 3-45
3.3.1 Major Sources of Information .................................................... 3-45
3.3.2 Public Water System Characteristics ......................................... 3-47
3.3.3 Financial Characteristics of Drinking Water Treatment
Systems ...................................................................................... 3-52
3.3.4 Customer Profile ........................................................................ 3-63
3.4 References .............................................................................................. 3-73
ii

Drinking Water Industry Report Contents
(Continued)
Page
SECTION 4 CURRENT STATE NPDES PERMIT REQUIREMENTS FOR WATER TREATMENT
PLANT RESIDUALS ................................................................................................ 4-1
4.1 Overview of State and Federal NPDES Regulatory Requirements for
Water Treatment Plants............................................................................ 4-1
4.2 Summary of Current Pollutant Limitations and Requirements for
Water Treatment Plants: General and Individual Permits ....................... 4-7
4.3 References .............................................................................................. 4-11
SECTION 5 SOURCE WATER QUALITY .................................................................................... 5-1
5.1 Factors That Influence Source Water Quality ......................................... 5-2
5.2 Comparison of Ground Water and Surface Water Quality ...................... 5-3
5.3 Source Water Protection Under the SDWA............................................. 5-4
5.4 References ................................................................................................ 5-5
SECTION 6 SOURCE WATER TREATMENT TECHNOLOGIES ...................................................... 6-1
6.1 Conventional Filtration, Direct Filtration, and Filtration Only ................ 6-2
6.1.1 Presedimentation .......................................................................... 6-4
6.1.2 Coagulation, Flocculation, and Sedimentation ............................ 6-4
6.1.3 Filtration ....................................................................................... 6-5
6.2 Precipitative (Lime) Softening ................................................................. 6-7
6.3 Membrane Separation .............................................................................. 6-8
6.3.1 Reverse Osmosis and Nanofiltration ........................................... 6-9
6.3.2 Microfiltration and Ultrafiltration .............................................. 6-10
6.3.3 Electrodialysis and Electrodialysis Reversal ............................. 6-11
6.4 Ion Exchange ......................................................................................... 6-12
6.5 Adsorptive Media—Activated Carbon .................................................. 6-13
6.6 Disinfection ............................................................................................ 6-14
6.6.1 Disinfection with Chlorine (Chlorination) ................................. 6-15
6.6.2 Disinfection with Chlorine Dioxide ........................................... 6-16
6.6.3 Disinfection with Chloramines (Chloramination) ..................... 6-17
6.6.4 Ozone Disinfection .................................................................... 6-17
6.6.5 Ultraviolet Light Disinfection .................................................... 6-18
6.7 Other Chemical Additions ..................................................................... 6-18
6.7.1 Corrosion and Scale Control ...................................................... 6-19
6.7.2 Solids Removal Using Sequestering Agents.............................. 6-19
6.7.3 pH Adjustment ........................................................................... 6-20
6.7.4 Water Additives ......................................................................... 6-20
6.8 References .............................................................................................. 6-20
SECTION 7 TYPES OF RESIDUALS PRODUCED BY SOURCE WATER TREATMENT ..................... 7-1
7.1 Presedimentation ...................................................................................... 7-1
7.2 Residuals from Coagulation, flocculation, and sedimentation ................ 7-2
7.3 Residuals from Precipitative (Lime) Softening ....................................... 7-4
7.4 Residuals from Filtration ......................................................................... 7-6
7.4.1 Filters (non-membrane) ............................................................... 7-6
7.4.2 Low-Pressure Membranes ........................................................... 7-7
iii

Drinking Water Industry Report Contents
(Continued)
Page
7.5 Residuals from Membrane Desalination .................................................. 7-9
7.6 Residuals from Ion Exchange ................................................................ 7-12
7.7 Residuals from Adsorption (Activated Carbon) .................................... 7-13
7.8 References .............................................................................................. 7-14
SECTION 8 POLLUTANTS IN WATER TREATMENT PLANT RESIDUALS ..................................... 8-1
8.1 Overview of Pollutants in Water Treatment Plant Residuals .................. 8-3
8.2 Solids In Water Treatment Plant Residuals ............................................. 8-4
8.3 Priority and Nonconventional Metals In Water Treatment Plant
Residuals .................................................................................................. 8-5
8.3.1 Aluminum and Iron ...................................................................... 8-9
8.3.2 Arsenic ......................................................................................... 8-9
8.3.3 Calcium and Sodium .................................................................... 8-9
8.3.4 Fluoride ...................................................................................... 8-10
8.3.5 Manganese and Potassium ......................................................... 8-10
8.3.6 Additional Metals with DMR Data ............................................ 8-10
8.4 WTP Pollutants from Disinfection......................................................... 8-11
8.4.1 Chemistry of Chlorine Disinfection ........................................... 8-12
8.4.2 Residual Disinfectants in Finished Drinking Water .................. 8-14
8.4.3 Disinfection By-Products ........................................................... 8-14
8.5 Parameters Measuring Organic Matter and Oxygen in the Water In
WTP Residuals....................................................................................... 8-15
8.5.1 Biochemical Oxygen Demand ................................................... 8-15
8.5.2 Dissolved Oxygen ...................................................................... 8-16
8.6 Other Pollutants in WTP ........................................................................ 8-17
8.6.1 Chloride...................................................................................... 8-17
8.6.2 Nitrogen ..................................................................................... 8-17
8.6.3 pH ............................................................................................... 8-18
8.6.4 Phosphorus ................................................................................. 8-18
8.6.5 Radionuclides ............................................................................. 8-18
8.7 References .............................................................................................. 8-19
SECTION 9 WATER TREATMENT PLANT POLLUTANT DISCHARGE ESTIMATES ....................... 9-1
9.1 Data Sources for the Pollutant Loadings Analysis .................................. 9-3
9.2 Methodology to Estimate Pollutant Loadings Using Model Plants ......... 9-4
9.2.1 Model Plant Development ........................................................... 9-4
9.2.2 Estimation of Model Plant Pollutant Loadings ............................ 9-6
9.3 Model Plant Concentration Estimation .................................................... 9-8
9.3.1 Selection of Pollutant Parameters for Pollutant Loadings
Analysis........................................................................................ 9-8
9.3.2 Development of Long-Term Average Concentrations for
Pollutants.................................................................................... 9-12
9.3.3 DMR Data Limitations .............................................................. 9-16
9.4 Model Plant Flow Rate Estimation ........................................................ 9-17
9.4.1 Review of DMR and Survey Data ............................................. 9-18
iv

Drinking Water Industry Report Contents
(Continued)
Page
9.4.2 Model Plant Effluent Flow Rate Results ................................... 9-19
9.5 Results of the Pollutant Loadings Estimate for Model Plants ............... 9-20
9.6 National Pollutant Discharge Estimates................................................. 9-39
9.7 References .............................................................................................. 9-43
SECTION 10 POTENTIAL SCOPE OF ENVIRONMENTAL IMPACTS OF POLLUTANT DISCHARGES 10-1
10.1 Review of Publicly Available Information ............................................ 10-1
10.2 Summary of Environmental Impact of WTP Residuals by Pollutant .... 10-2
10.2.1 Environmental Impact of Solids ................................................ 10-2
10.2.2 Environmental Impact of Metals ............................................... 10-3
10.2.3 Environmental Impact of Chlorine and Chloramines ................ 10-3
10.2.4 Environmental Impact of Oxygen Demand ............................... 10-4
10.2.5 Environmental Impact of Chlorides ........................................... 10-4
10.2.6 Environmental Impact of Nitrogen ............................................ 10-5
10.2.7 Environmental Impact of pH Changes ....................................... 10-6
10.2.8 Environmental Impact of Phosphorus ........................................ 10-6
10.2.9 Environmental Impact of Radionuclides ................................... 10-7
10.3 References .............................................................................................. 10-7
SECTION 11 TECHNOLOGIES AND PRACTICES FOR PREVENTING, TREATING, DISPOSING OF,
AND
DISCHARGING SOURCE WATER TREATMENT RESIDUALS ............................ 11-1
11.1 Pollution Prevention and Waste Reduction ........................................... 11-2
11.1.1 Optimize Intake Water Conditions ............................................ 11-4
11.1.2 Optimize Filter Media ................................................................ 11-4
11.1.3 Optimize pH to Reduce Coagulant Chemicals .......................... 11-5
11.1.4 Reduce Softening Chemicals by Monitoring Source Water
Hardness ..................................................................................... 11-6
11.1.5 Return Backwash Water and Filter-to-Waste to the Head of
the Source Water Treatment Plant for Reuse ............................. 11-6
11.1.6 Reuse of Precipitative Softening Chemicals .............................. 11-7
11.1.7 Recovery of Treatment Chemicals ............................................. 11-8
11.2 Residuals Treatment............................................................................. 11-10
11.2.1 Solids Removal (Separation of Solids and Water) .................. 11-11
11.2.2 Chemical Precipitation ............................................................. 11-20
11.2.3 Increased Oxygen Content by Aeration ................................... 11-20
11.2.4 Dechlorination.......................................................................... 11-20
11.2.5 pH Adjustment ......................................................................... 11-21
11.2.6 Nonwater Quality Environmental Impact Considerations ....... 11-21
11.3 Disposal Practices for Treatment Residuals ........................................ 11-23
11.3.1 Land Application of Residuals................................................. 11-23
11.3.2 Disposal of Residuals to Landfills or Deep Injection Wells .... 11-24
11.4 Wastewater Discharges of Treatment Residuals .................................. 11-24
11.5 References ............................................................................................ 11-26
v

Drinking Water Industry Report Contents
(Continued)
Page
SECTION 12 TREATMENT TECHNOLOGY COST CONSIDERATIONS FOR RESIDUALS
THICKENING AND DEWATERING ......................................................................... 12-1
12.1 Residuals Thickening And Dewatering Treatment Train ...................... 12-1
12.2 Cost Data Sources Identified ................................................................. 12-4
12.2.1 Drinking Water Treatment Technology Review Group ............ 12-4
12.2.2 AWWA 2008 Cost Estimates .................................................... 12-6
12.2.3 EPA’s Work Breakdown Structure (WBS) Cost Models .......... 12-7
12.3 Treatment Units: Description and Capacity ........................................... 12-8
12.3.1 Typical Ranges of Solids Content and Flow in Residuals
from Conventional Filtration and Softening Plants ................... 12-8
12.3.2 Spent Filter Backwash Equalization and Clarifier Capacity .... 12-10
12.3.3 Gravity Thickener Capacity ..................................................... 12-12
12.3.4 Sludge Dewatering Centrifuges and Equalization Tanks ........ 12-13
12.3.5 Ancillary Equipment ................................................................ 12-14
12.4 Costs to Install And Operate Residuals Treatment Systems ................ 12-14
12.4.1 Capital Costs for Treatment Units ........................................... 12-15
12.4.2 Indirect Capital Costs ............................................................... 12-16
12.4.3 Annual Operating Costs ........................................................... 12-16
12.4.4 Additional Costs that Vary Between WTPs............................. 12-16
12.5 References ............................................................................................ 12-20
SECTION 13 ECONOMIC ACHIEVABILITY METHODOLOGY ...................................................... 13-1
13.1 Introduction ............................................................................................ 13-1
13.2 A Methodology for Determining the Economic Achievability of Best
Professional Judgment Effluent Limitations for A Public Water
System .................................................................................................... 13-2
13.2.1 Estimate Increase in Water Rates to Household Customers ...... 13-4
13.2.2 Estimate Increase in Annual Water Service Cost for
Household Customers .............................................................. 13-10
13.2.3 Estimate Number and Percentage of Households, by Water
System, for which the Annual Household Water Service Cost
Increase Exceeds a Percent of Income Achievability
Threshold ................................................................................. 13-12
13.2.4 Assessing the Impact of Rate Structure on the Achievability
Determination .......................................................................... 13-19
13.3 References ............................................................................................ 13-20
SECTION 14 GLOSSARY, ACRONYMS, AND ABBREVIATIONS .................................................. 14-1
APPENDIX A: SURVEY DESIGN AND CALCULATION OF NATIONAL ESTIMATES
APPENDIX B: COMPOSITION OF COMMON DRINKING WATER TREATMENT CHEMICALS
ILLUSTRATING PRODUCTION IMPURITIES
APPENDIX C: POTW PERCENT REMOVALS
vi

Drinking Water Industry Report Contents
(Continued)
Page
APPENDIX D: TOXIC WEIGHTING FACTORS (TWFS)
APPENDIX E: NATIONAL ESTIMATES: WATER TREATMENT PLANT COUNTS FOR POLLUTANT
LOADINGS ESTIMATES
vii

Drinking Water Industry Report List of Tables
LIST OF TABLES
Page
Table 2-1. EPA Site Visits to Drinking Water Treatment Plants .......................................... 2-3
Table 3-1. Discharge Status for Water Treatment Plants Serving More than 10,000
People ................................................................................................................... 3-2
Table 3-2. Industry National Estimates: Numbers of WTPs and Systems ............................ 3-6
Table 3-3. Number of People Served per WTP in 2006 (National Estimates Based on
Responses to Question 2b) ................................................................................... 3-9
Table 3-4. Estimated Water Production per WTP in 2006 (National Estimates Based
on Responses to Questions 2b and 2c) ............................................................... 3-10
Table 3-5. Operating Days per WTP in 2006 (National Estimates Based on
Responses to Question 2c) ................................................................................. 3-11
Table 3-6. WTP Age (National Estimates Based on Responses to Question 2d) ............... 3-12
Table 3-7. Estimated Number of WTPs Using Presedimentation (National Estimates
Based on Responses to Question 2f) .................................................................. 3-16
Table 3-8. Estimated Numbers of WTPs Using Various Primary Disinfection
Methods (National Estimates Based on Responses to Question 2f) .................. 3-17
Table 3-9. Disinfection Residuals in Filter Backwash and Filter-to-Waste (National
Estimates Based on Responses to Question 2f) ................................................. 3-18
Table 3-10. Primary Disinfectants (National Estimates Based on Responses to
Question 2f) ....................................................................................................... 3-19
Table 3-11. Residuals Treatment Methods (National Estimates Based on Responses to
Question 2h) ....................................................................................................... 3-22
Table 3-12. Pollution Prevention Methods (National Estimates Based on Responses to
Question 2i) ........................................................................................................ 3-23
Table 3-13. Estimated Numbers of WTPs Using Direct, Indirect, or Zero Residuals
Discharge Practices (National Estimates Based on Responses to Question
2k) ...................................................................................................................... 3-30
Table 3-14a. Estimated Numbers of WTPs by Types of Residuals Discharged and
Discharge Practice (National Estimates Based on Responses to
Question 2k) ....................................................................................................... 3-31
viii

Drinking Water Industry Report List of Tables
(Continued)
Page
Table 3-14b. Estimated Numbers of WTPs by Types of Residuals Discharged and
Discharge Practice (National Estimates Based on Responses to
Question 2k) ....................................................................................................... 3-32
Table 3-15. Estimated Number of WTPs by Discharge Frequency for Direct and
Indirect Discharges (National Estimates Based on Responses to
Question 2k) ....................................................................................................... 3-33
Table 3-16. Estimated Number of Batch and Emergency Dischargers by Direct-
Discharging WTPs (National Estimates Based on Responses to
Question 2k) ....................................................................................................... 3-34
Table 3-17. Estimated Numbers of WTPs Directly Discharging to Various Types of
Receiving Waters (National Estimates Based on Responses to
Question 2k) ....................................................................................................... 3-35
Table 3-18. Estimated Number of WTPs with Indirect Discharge and Release
Volumes for Continuous Discharges (National Estimates Based on
Responses to Question 2k) ................................................................................. 3-36
Table 3-19. Estimated Number of WTPs with Indirect Discharge and Release Volumes
for Batch Discharges (National Estimates Based on Responses to
Question 2k) ....................................................................................................... 3-37
Table 3-20. Estimated Number of WTPs Employing Various Zero Discharge Disposal
Methods (National Estimates Based on Responses to Question 2k) ................. 3-38
Table 3-21. Estimated Number of WTPs Using Copper Sulfate and Application Rate
(National Estimates Based on Responses to Question 3) .................................. 3-41
Table 3-22. Estimated Number of WTPs Using Chelated Copper Complexes and
Application Rate (National Estimates Based on Responses to Question 3) ...... 3-42
Table 3-23. Estimated Number of WTPs Using Copper Sulfate and Amount of
Metallic Copper Used in Pounds (National Estimates Based on Responses
to Question 3) ..................................................................................................... 3-43
Table 3-24. Estimated Number of WTPs Using Chelated Copper Complexes and .............. 3-44
Table 3-25. Number of PWSs and Total Population Served by System Type, SDWIS ....... 3-47
Table 3-26. Summary of the Number of PWSs by System Type and Size, SDWIS ............ 3-47
Table 3-27. Number of Systems that Report Water Sales to Different Customer
Categories, DWT Industry Questionnaire.......................................................... 3-48
Table 3-28. Number of Water Systems by Ownership Type and Size, SDWIS ................... 3-49
ix

Drinking Water Industry Report List of Tables
(Continued)
Page
Table 3-29. Number of Water Systems by Water Source and System Size, SDWIS ........... 3-50
Table 3-30. Summary of CWSs by Water Source and Population Served, CWSS .............. 3-52
Table 3-31. Reported 2006 Water Quantity Sold (MGY), per System, DWT Industry
Questionnaire ..................................................................................................... 3-54
Table 3-32. Summary of Annual CWS Revenues by Ownership Type ($1,000), CWSS .... 3-54
Table 3-33. Summary of Total Revenues of CWSs that Discharge ($/1,000 gallons).......... 3-55
Table 3-35. Reported 2006 Water Sales Revenue per Volume, per System, DWT
Industry Questionnaire ....................................................................................... 3-56
Table 3-36. Average System Expenses and Expense Breakdown by Major Category,
CWSS ................................................................................................................. 3-58
Table 3-37. Summary of Total Expenses by System Size and Ownership Type
($/1,000 gallons produced), CWSS ................................................................... 3-58
Table 3-38. Reported 2006 Total Expenses, per System, DWT Industry Questionnaire...... 3-60
Table 3-39. Reported 2006 Expenses per MGY, Total and Operating, per System,
DWT Industry Questionnaire ............................................................................. 3-61
Table 3-40. Reported 2006 Hourly and Total Wages for All Employees, per System,
DWT Industry Questionnaire ............................................................................. 3-62
Table 3-41. Number and Percentage of CWSs Serving Different Customer Types,
CWSS ................................................................................................................. 3-64
Table 3-42. Amount of Water Delivered by Customer and Ownership Type and
System Size (billion gallons; 2000), CWSS ...................................................... 3-65
Table 3-43. Reported 2006 Water Sales to Residential Customers, by System, DWT
Industry Questionnaire ....................................................................................... 3-66
Table 3-44. Revenues by Customer Type (in million $), CWSS .......................................... 3-67
Table 3-45. Median Revenue per 1000 Gallons of Water Delivered by Customer Type,
Ownership Type, and System Size ($/1000 gallons), CWSS ............................ 3-68
Table 3-46. Summary of Median Annual Residential Water Bill, CWSS ............................ 3-69
Table 3-47. Number of Systems Using Various Billing Methods for All Customers,
2006, DWT Industry Questionnaire ................................................................... 3-70
x

Drinking Water Industry Report List of Tables
(Continued)
Page
Table 3-48. Number and Percentage of Systems with Lower Rates for Low- or Fixed-
Income Households, CWSS ............................................................................... 3-71
Table 3-49. Reported 2006 Household Participation in System Assistance Programs
and Income Requirements, DWT Industry Questionnaire ................................. 3-72
Table 3-50. Number of Households with Lower Rates and Range of Qualifying
Household Incomes, CWSS ............................................................................... 3-72
Table 4-1. Summary of Permit Information in the 2004 Permit Compliance System .......... 4-2
Table 4-2. Wastewater Discharges from WTPs Covered by General Permits ...................... 4-6
Table 4-3. Range of Pollutant Limitations From a Sample of General and Individual
NPDES Permits .................................................................................................... 4-8
Table 5-1. Common Source Water Contaminants and Sources ............................................ 5-1
Table 7-1. Typical Chemical Coagulation Sludge Volumes ................................................. 7-4
Table 7-2. Typical Lime Softening Sludge Volumes ............................................................ 7-5
Table 7-3. Typical Characteristics of Low-Pressure Membrane Backwash Residuals ......... 7-8
Table 7-4. Typical Characteristics of Spent Low-Pressure Membrane Chemical
Cleaning Solutions ............................................................................................... 7-9
Table 7-5. Membrane Desalination: Typical Target Contaminants by Source Water ........ 7-11
Table 7-6. Typical Membrane Desalination System (RO and NF) Design Parameters ...... 7-11
Table 7-7. Typical Ion Exchange Concentrate Volumes .................................................... 7-13
Table 7-8. Typical Chemical Concentrations in Ion Exchange Waste Concentrate ........... 7-13
Table 8-1. Priority Pollutant List
a
........................................................................................ 8-2
Table 8-2. Evaluation of Priority and Nonconventional Metals in Water Treatment
Plant Residuals ..................................................................................................... 8-6
Table 9-1. Pollutants Included in the Loadings Estimates .................................................... 9-9
Table 9-2. Type of Source Water Treatment and Residuals in Place (Solid/Water
Separation) for WTPs with DMR Data .............................................................. 9-11
Table 9-3. Type of Source Water Treatment and Residuals in Place (Dechlorination)
for WTPs with DMR Data ................................................................................. 9-12
xi

Drinking Water Industry Report List of Tables
(Continued)
Page
Table 9-4. Long-Term Average Concentrations from DMR Data by Source Water
Treatment Type and Residuals Treatment (mg/L) ............................................. 9-14
Table 9-5. Long-Term Average Concentrations for Pollutants Resulting from
Disinfection with Chlorine ................................................................................. 9-16
Table 9-6. Model Plant Effluent Flow Rates ....................................................................... 9-19
Table 9-7. Model Plant Pollutant Loadings by Source Water Treatment Type and
Residuals Treatment Type (With and Without Solid/Water Separation) for
Direct and Indirect (Pass Through) Discharges: Population Served of
10,001 to 50,000 People..................................................................................... 9-21
Table 9-8. Model Plant Pollutant Loadings by Source Water Treatment Type and
Residuals Treatment Type (With and Without Solid/Water Separation) for
Direct and Indirect (Pass Through) Discharges: Population Served of
50,001 to 100,000 People................................................................................... 9-24
Table 9-9. Model Plant Pollutant Loadings by Source Water Treatment Type and
Residuals Treatment Type (With and Without Solid/Water Separation) for
Direct and Indirect (Pass Through) Discharges: Population Served of
100,001 to 500,000 People................................................................................. 9-28
Table 9-10. Model Plant Pollutant Loadings by Source Water Treatment Type and
Residuals Treatment Type (With and Without Solid/Water Separation) for
Direct and Indirect (Pass Through) Discharges: Population Served of
More than 500,000 People ................................................................................. 9-31
Table 9-11. Model Plant Pollutant Loadings for WTPs Performing Chlorination by
Source Water Treatment Type and Residuals Treatment Type (With and
Without Dechlorination) for Direct and Indirect (Pass Through)
Discharges: Population Served of 10,001 to 100,000 People ............................ 9-34
Table 9-12. Model Plant Pollutant Loadings for WTPs Performing Chlorination by
Source Water Treatment Type and Residuals Treatment Type (With and
Without Dechlorination) for Direct and Indirect (Pass Through)
Discharges: Population Served Greater than 100,000 People ........................... 9-37
Table 9-13. Pollutant Loadings
a
for WTPs: National Estimates by Source Water
Treatment Type and Pollutant ............................................................................ 9-41
Table 9-14. Pollutant Loadings
a
for WTPs Serving More than 10,000 People:
National Estimate by Source Water Treatment Type and WTP Size (as
Population Served) ............................................................................................. 9-43
Table 11-1. Distribution of Residuals Treatment Technologies at Drinking Water
Treatment Plants .............................................................................................. 11-10
xii

Drinking Water Industry Report List of Tables
(Continued)
Page
Table 11-2. Comparison of Solids Removal Technologies: Solids Concentration After
Treatment by Residuals Type .......................................................................... 11-12
Table 11-3. Laboratory Results for Mechanical Dewatering Operations for Various
Drinking Water Treatment Residuals .............................................................. 11-17
Table 12-1. Available Residuals Management Cost Equations ............................................ 12-6
Table 12-2. Ranges of Residuals Production Estimated for AWWA 2008 Study ................ 12-9
Table 12-3. SFBW Equalization Basin Capacity ................................................................ 12-11
Table 12-4. Indirect Cost Factors and Selected Unit Costs for WTP Residuals
Treatment System Planning ............................................................................. 12-18
Table 13-1. Example of Income Distribution from the 2000 U.S. Census ......................... 13-13
Table 13-2. Example of Income Distribution Provided by the U.S. Census With
Ranges Updated to Current Year (10% increase in income) ........................... 13-14
Table 13-3. Example of Income Distribution Provided by the U.S. Census With
Ranges and Number of Households Updated to Current Year (10%
increase in income and 3% increase in population) ......................................... 13-15
Table 13-4. Example of the Calculation of Number and Percent of Households above
an Achievability Threshold (1.0% of Median Household Income) ................. 13-18
Table 13-5. Example of the Calculation of Number and Percent of Households above
an Achievability Threshold (1.0% of Median Household Income)
assuming a Lifeline Rate Structure for Income Below $16,500 ...................... 13-20
xiii

Drinking Water Industry Report List of Figures
LIST OF FIGURES
Page
3-1 Question 2b: Population Served by the WTP in 2006 ......................................... 3-5
3-2 Question 2e: Source Water Type ......................................................................... 3-5
3-3 Questions 2b-d: WTP Operating Characteristics ................................................. 3-8
3-4 Question 2f: Source Water Treatment and Chemical Addition:
Presedimentation ................................................................................................ 3-13
3-5 Question 2f: Source Water Treatment and Chemical Addition: Primary
Disinfection and Dechlorination ........................................................................ 3-14
3-6 Question 2f: Source Water Treatment and Chemical Addition:
Disinfection Residuals ....................................................................................... 3-14
3-7 Question 2f: Source Water Treatment and Chemical Addition: Primary
Disinfectant ........................................................................................................ 3-15
3-8 Question 2h: Residuals Treatment ..................................................................... 3-20
3-9 Question 2i: Pollution Prevention ...................................................................... 3-21
3-10 Question 2k: Residuals Discharge Method ........................................................ 3-25
3-11 Question 2k: Type of Residuals Discharged ...................................................... 3-26
3-12 Question 2k: Frequency of Residuals Discharge ............................................... 3-27
3-13 Question 2k: Direct Discharge—Continuous, Batch or Emergency and
Type of Receiving Stream ................................................................................. 3-27
3-14 Question 2k: Indirect Discharge—Continuous, Batch or Emergency and
Volume Discharged ........................................................................................... 3-28
3-15 Question 2k: Zero Discharge Methods .............................................................. 3-29
3-16 Question 3: Use of Copper-Based Chemicals to Treat Source Water ............... 3-40
6-1 Typical Conventional Filtration Treatment Plant Flow Diagram (U.S.
EPA, 2002a) ......................................................................................................... 6-3
6-2 Reverse Osmosis Cross-Flow Membrane (The Merit Partnership, 2002) ......... 6-10
6-3 Ozone Disinfection Process Flow Diagram (U.S. EPA, 1986).......................... 6-18
xiv

Drinking Water Industry Report List of Figures
(Continued)
Page
7-1 Residuals from Source Water Solids Removal (U.S. EPA/ACSE/AWWA,
1996) .................................................................................................................... 7-2
7-2 Residuals from Precipitative Softening WTP ...................................................... 7-6
7-3 Residuals from Membrane Desalination ............................................................ 7-10
8-1 Chemistry of Compounds Resulting from Chlorine Disinfection (CDC,
2006; Block, 2000)............................................................................................. 8-12
11-1 WTP Pollution Prevention and Waste Reduction Practices in the U.S. in
2006.................................................................................................................... 11-3
11-2 Gravity Thickener (U.S. EPA, 2003) ............................................................... 11-14
11-3 Belt Filter Press (U.S. EPA, 2000a) ................................................................. 11-16
11-4 Sand Drying Bed Section (U.S. EPA, ASCE, and AWWA, 1996) ................. 11-19
12-1 Residuals Treatment Technology Train ............................................................. 12-3
xv

SECTION 1
INTRODUCTION
The U.S. Environmental Protection Agency (EPA) completed a review of
discharges from water treatment plants (WTPs). The purpose of this report is to summarize the
data collected during this review (principally covered in Sections 2, 3, 9, 10, and 11) and to serve
as a technical resource to permit writers (primarily covered in Sections 4 through 8 and Sections
12 and 13).
EPA selected the drinking water treatment (DWT) industry for a rulemaking as
part of its 2004 Biennial Effluent Limitations and Guidelines Program planning process. EPA is
not at this time continuing its effluent guidelines rulemaking for the DWT industry. In the 2004
Plan, EPA announced that it would begin development of a regulation to control the pollutants
discharged from medium and large DWT plants. See 69 FR 53720 (September 2, 2004). Based
on a preliminary study and on public comments, EPA was interested in the potential volume of
discharges associated with drinking water facilities. The preliminary data were not conclusive,
and the Agency proceeded with additional study and analysis of treatability, including an
industry survey. After considering extensive information about the industry, its treatment
residuals, wastewater treatment options, and discharge characteristics, and after considering
other priorities, EPA has suspended work on this rulemaking.
The DWT industry serves to provide potable water to its customers. The DWT
industry falls under Standard Industrial Classification (SIC) code 4941, which crosswalks with
North American Industry Classification System (NAICS) code 22131. In addition to drinking
water, SIC code 4941 includes other water supply plants—those that treat water for use in
commercial and industrial applications. NAICS code 22131 includes all of SIC code 4941 plus
irrigation systems (defined by SIC code 4971). For this industry review, EPA focused on
drinking water systems that serve more than 10,000 people. Most systems that serve more than
10,000 people are defined as community water systems (CWSs) under the Safe Drinking Water
Act. CWSs serve the same customer base year round (e.g., city water authority).
1-1

Drinking Water Industry Report Section 1 – Introduction
Drinking water systems may obtain their water supply either directly from the
source (e.g., river, lake, reservoir for surface water sources or via wells for ground water sources)
or may purchase from wholesalers. Systems may treat the source water (i.e., intake water) prior
to distribution or only provide delivery of the drinking water. If the system treats the source
water prior to delivery, the system operates one or more WTPs.
Based on EPA’s industry survey, 2,151 WTPs serve populations greater than
10,000 people and generate waste streams from the treatment of source water. Sixty-eight
percent (1,464 plants) serve between 10,001 and 50,000 people, and on average produce 3.49
million gallons per year of finished drinking water. The remaining 32 percent (688 plants) serve
more than 50,000 people and produce between three and 55,000 million gallons per year of
finished drinking water. The average drinking water production per day for the 688 WTPs is
23.46 million gallons. For all 2,151 WTPs, the average quantity of drinking water produced per
person per year is over 53,000 gallons.
During the treatment of source water, WTPs remove contaminants that are
unhealthy or undesirable for consumption. The generated waste streams are treatment residuals.
EPA estimates that approximately 31 percent of the 2,151 WTPs directly discharge to surface
water. An additional 7 percent discharge both directly to surface water and indirectly by
transferring residuals to POTWs. The discharge of treatment residuals is the issue of interest in
this industry review.
Since 2004, EPA has conducted site visits, completed an industry survey, worked
with the industry (e.g., American Water Works Association), and collected other information.
EPA produced this technical report to summarize the collected information and our analysis.
Section 2.0 summarizes EPA’s activities to identify and collect data as part of the industry
review. Subsequent sections of this report summarize analyses conducted using data from these
sources. In particular:
• Section 3.0 characterizes the water treatment industry by size of
population served, primary water source (e.g., ground, surface), treatment
method(s) used (e.g., precipitative softening, conventional filtration,
membrane desalination, ion exchange), and discharges. It provides an
1-2

Drinking Water Industry Report Section 1 – Introduction
overview of financial characteristics of the industry and a discussion of
water consumption and rates.
• Section 4.0 analyzes state permit requirements including both general
and individual permits, pollutants regulated (e.g., aluminum, iron,
manganese, pH, settleable solids), range of pollutant limitations, and
special requirements for systems based on treatment technologies used.
• Section 5.0 discusses source water quality and the factors that
influence it. Influencing factors include naturally-occurring attributes
(climate, geology, soil type, land cover, hydrology, precipitation and
runoff, and wildlife) and man-made attributes (land management practices
and runoff or upstream discharge from point and nonpoint sources).
• Section 6.0 reviews source water treatment technologies including
conventional filtration, direct filtration, and filtration only; precipitative
softening; membrane separation; ion exchange; activated carbon;
disinfection; and other chemical additions.
• Section 7.0 examines residuals produced by each of the source water
treatment technologies. Residuals generated by WTPs include solids
contaminants removed during precipitative softening (softening sludge);
solids and contaminants removed during coagulation, flocculation, and
sedimentation (coagulation sludge); filter backwash water; concentrates
from membrane desalination; spent membrane cleaning solutions; ion
exchange waste concentrates; and regeneration wastes from adsorption
processes.
• Section 8.0 discusses pollutants in drinking water treatment residuals
including suspended and dissolved solids, metals (e.g., aluminum, iron,
lead, and manganese), disinfection by-products (e.g., trihalomethanes and
haloacetic acids), and other pollutants.
• Section 9.0 provides EPA’s national estimate of pollutant discharges
from WTPs. In addition to the estimate, this section describes data
sources and methodology used; selection of pollutants to include in the
loadings estimates; development of long-term averages for pollutants; and
pollutant loadings estimates for model plants.
• Section 10.0 describes the potential environmental impacts of
pollutant discharges. EPA completed a literature review to gather data on
potential environmental impacts from discharges of WTP residuals. The
majority of studies focused on discharges of lime sludge and alum sludge
from lime softening and conventional filtration plants. This section
summarizes EPA’s review of environmental impacts from WTP
discharges.
1-3

Drinking Water Industry Report Section 1 – Introduction
• Section 11.0 discusses best management practices for handling,
minimizing, and preventing source water treatment residuals.
Example best management practices include source reduction activities
(e.g., optimization of surface water intake to reduce suspended solids,
optimization of filter media for finished water), and treatment of residuals,
recycling and reuse of residuals, and land application of residuals.
• Section 12.0 reviews cost considerations for residuals thickening and
dewatering. Technology options exist to reduce discharges of residuals.
This section examines the factors that affect the cost of installing and
operating residuals treatment systems for conventional filtration (i.e.,
coagulation and filtration) and lime softening plants.
• Section 13.0 discusses the methodology to assess economic
achievability. EPA outlines an approach to determine the economic
achievability of installing new technology to treat residuals at WTPs.
• Section 14.0 includes a glossary, acronyms, and abbreviations used in
this report.
1-4

SECTION 2
DATA SOURCES
EPA conducted a number of data collection activities and reviewed a number of
data sources in support of the drinking water treatment (DWT) industry review. Section 2.1
describes EPA’s site visits and Section 2.2 describes EPA’s industry questionnaire. Section 2.3
discusses ground water and drinking water data collected by EPA under the Safe Drinking Water
Act (SDWA). Section 2.4 presents other information collection activities and data sources,
including literature searches, National Pollutant Discharge Elimination System (NPDES)
permits, NPDES Discharge Monitoring Reports (DMRs), other EPA data sources, and industry
data. Section 2.5 describes EPA’s outreach efforts through stakeholder meetings and Section 2.6
describes the DWT technology review.
2.1 SUMMARY OF EPA’S WATER TREATMENT PLANT SITE VISITS
EPA conducted 14 engineering site visits to drinking water treatment plants
(WTPs) and a technology vendor research and manufacturing plant to gather information about
industry operations, sources of residuals, residuals management practices, and residuals
treatment technologies
. EPA used information collected from literature searches and contact with
trade association members to identify representative WTPs for site visits. In general, EPA
considered the following when selecting WTPs to visit:
• Size of plant (medium and large plants);
• Geographic location (variable source water qualities); and
• Residuals management practices (for treatment technologies that generate
residuals).
Plant-specific selection criteria are contained in site visit reports prepared for each
plant visited by EPA. During the site visits, EPA collected the following information:
• Plant description (e.g., size, production volume, location);
• Source water treatment technologies;
2-1

Drinking Water Industry Report Section 2 – Data Sources
• Residuals generation, treatment, and management; and
• Permitting requirements.
This information is documented in the site visit report for each WTP visited. Table 2-1 lists the
site visits EPA performed and the document control number (DCN) for the site visit report.
2.2 EPA DWT INDUSTRY QUESTIONNAIRE
2.2.1 Overview of Industry Questionnaire
EPA used an industry questionnaire to collect site-specific technical and
economic information for Community Water Systems (CWSs) and WTPs operated by the
systems. CWSs are drinking water systems that serve the same customer base year round (e.g.,
city water authority). The majority of drinking water is distributed by CWSs.
EPA published a notice in the Federal Register on July 5, 2005 (70 FR 38675)
announcing its intent to submit a survey Information Collection Request (ICR) to the Office of
Management and Budget (OMB). The notice requested comment on the draft ICR and two draft
survey questionnaires (screener and detailed). EPA revised the survey questionnaires as a result
of the public comments received, which included comments from the Association of
Metropolitan Water Agencies (AMWA) and American Water Works Association (AWWA).
Among other changes EPA collapsed the two questionnaires into one. EPA subsequently
obtained OMB approval to administer one survey questionnaire (71 FR 41012, July 19, 2006).
2-2

Drinking Water Industry Report Section 2 – Data Sources
Table 2-1. EPA Site Visits to Drinking Water Treatment Plants
Water Treatment Plant (WTP)
Name
Date of EPA Site
Visit
Type of Source Water Treatment
Type of Residuals Treatment
Site Visit
Report DCN
James J. Corbalis WTP
(Fairfax County, VA)
November 3, 2004
Conventional filtration of surface water;
disinfection using chlorine and chloramines
Solids dewatering: gravity
thickening and plate and filter press;
Recycle water from dewatering
DW00178
Bexar Metropolitan Ultrafiltration
WTP
(San Antonio, TX)
November 18, 2004
Ultrafiltration with coagulation/sedimentation
of surface water; disinfection using chlorine
Equalization;
Evaporation ponds;
Recycle filter backwash
DW03706
Washington Aqueduct: Dalecarlia
WTP (Washington, DC)
November 30, 2004
Conventional filtration of surface water;
disinfection using chloramine
Dewatering facility is under
construction
DW03707
Rivanna Water and Sewer
Authority: South Rivanna WTP
(Charlottesville, VA)
March 31, 2005
Conventional filtration of surface water;
disinfection using chlorine
Equalization, clarification, and
recycling of wastewater;
Solids dewatering: belt filter press
DW03708
Rivanna Water and Sewer
Authority: Scottsvillle WTP
(Charlottesville, VA)
March 31, 2005
Conventional filtration of surface water;
disinfection using chlorine
Equalization, clarification, and
recycling of wastewater;
Settling in lagoons prior to discharge
Evitts Creek WTP
(Cumberland, MD)
July 14, 2005
Direct filtration of surface water, including use
of dissolved air flotation (DAF); disinfection
using chlorine (ammonia added to distribution
system to form chloramines)
Solids dewatering: thickening and
belt filter press
DW03709
F.B. Leopold Company
(Zelienople, PA)
July 15, 2005
Vendor research and manufacturing facility
DW00223
Fleur Drive WTP
(Des Moines, IA)
October 6, 2005
Source water: surface water (Aspects of this report are claimed by the facility to be
Confidential Business Information)
DW00918
Newport News Water Works: Lee
Hall Facility
(Newport News, VA)
October 7, 2005
Conventional filtration of surface water (with
DAF);
Reverse osmosis of ground water;
Disinfection of finished water from both plants
using chlorine or ozone
Equalization and gravity thickeners;
Thickening sludge treated off-site in
centrifuges
DW03710
City of Melbourne: Joe Mullins
Reverse Osmosis WTP
(Melbourne, FL)
October 14, 2005
Reverse osmosis of ground water; disinfection
of finished water using chlorine
Concentrate is degasified to remove
hydrogen sulfide and carbon
dioxide;
Acid is added to lower the pH;
Air injected prior to discharge to
increase dissolved oxygen levels
DW00903
2-3

Drinking Water Industry Report Section 2 – Data Sources
Table 2-1. EPA Site Visits to Drinking Water Treatment Plants
Water Treatment Plant (WTP)
Name
Date of EPA Site
Visit
Type of Source Water Treatment
Type of Residuals Treatment
Site Visit
Report DCN
City of Melbourne: John A.
Buckley Surface WTP
(Melbourne, FL)
October 14, 2005
Conventional filtration (activated carbon filters)
of surface water; disinfection of finished water
using chlorine
Equalization (filter backwash);
Solids dewatering: filter presses;
Wastewater recycled
DW00903
E.M. Johnson WTP
(Raleigh, NC)
October 17, 2005
Conventional filtration of surface water;
disinfection using chlorine (sodium
hypochlorite) and chloramine (at clear well)
Clarification of filter backwash;
Solids dewatering: gravity
thickening and belt filter press
DW00905
Val Vista WTP
(Mesa, AZ)
January 18, 2006
Conventional filtration of purchased water
(surface water); disinfection using chlorine
Filter backwash clarifiers;
Solids dewatering: gravity
thickeners and centrifuges
DW00891
Alvarado WTP
(San Diego, CA)
January 19, 2006
Conventional filtration of purchased and surface
water; disinfection using chlorine but plans to
introduce ozone disinfection
None: residuals returned to intake
reservoir (source water)
DW00907
Puerto Rico Aqueduct and Sewer
Authority (PRASA): Arecibo
WTP
August 8 – 10, 2006
Conventional filtration of surface water;
disinfection using chlorine
None
DW03711
PRASA: El Yunque WTP
August 8 – 10, 2006
Conventional filtration of surface water;
disinfection using chlorine
Sludge drying: vacuum-assisted
PRASA: Canovanas WTP
August 8 – 10, 2006
Conventional filtration of surface water;
disinfection using chlorine
Recycle
PRASA: Enrique Ortega (La
Plata) WTP
August 8 – 10, 2006
Conventional filtration of surface water;
disinfection using chlorine
Sludge drying: vacuum-assisted
PRASA: Los Filtros (Guaynabo)
WTP
August 8 – 10, 2006
Conventional filtration of surface water;
disinfection using chlorine
Sludge drying: vacuum-assisted
PRASA: Sergio Cuevas
Bustamante WTP
August 8 – 10, 2006
Conventional filtration of surface water;
disinfection using chlorine
Sludge drying: vacuum-assisted
Thames Water: Superaqueduct
WTP
August 8 – 10, 2006
Conventional filtration of surface water;
disinfection using chlorine
Solids dewatering: lagoon
2-4

Drinking Water Industry Report Section 2 – Data Sources
Table 2-1. EPA Site Visits to Drinking Water Treatment Plants
Water Treatment Plant (WTP)
Name
Date of EPA Site
Visit
Type of Source Water Treatment
Type of Residuals Treatment
Site Visit
Report DCN
Missouri American Water
Company: St. Joseph Plant
(St. Joseph, MO)
October 16, 2006
Precipitative (lime) softening of ground water;
disinfection using chlorine
Filter backwash recycled;
Settling basin prior to discharge
DW03772
Kansas City WTP
(Kansas City, MO)
October 17, 2006
Precipitative (lime) softening of surface and
ground water; disinfection using chloramine
None
Courtney Bend Water Plant
(Independence, MO)
October 17, 2006
Precipitative (lime) softening of ground water;
disinfection using chloramines
Filter backwash recycled
Boonville WTP
(Boonville, MO)
October 18, 2006
Direct filtration of surface water
None
Missouri American Water
Company: Jefferson City Plant
(Jefferson City, MO)
October 18, 2006
Precipitative (lime) softening of surface water
None
St. Louis Water Division:
Chain of Rocks WTP
(St. Louis, MO)
October 19, 2006
Precipitative (lime) softening of surface water;
disinfection using chlorine
None
St. Louis Water Division: Central
Plant (St. Louis, MO)
October 19, 2006
Precipitative (lime) softening of surface water;
disinfection using chlorine
None
St. Louis Water Division: Howard
Bend Plant (St. Louis, MO)
October 19, 2006
Precipitative (lime) softening of surface water;
disinfection using chlorine
None
St. Louis Water Division: PWSD
#2 (St. Louis, MO)
October 19, 2006
Precipitative (lime) softening and aeration of
ground water; disinfection using chlorine
None
St. Louis Water Division: North
Plant (St. Louis, MO)
October 19, 2006
Precipitative (lime) softening of surface water;
disinfection using chlorine
None
Illinois American Water
Company: Alton Plant (Alton, IL)
October 19, 2006
Conventional filtration of surface water,
disinfection using chloramines (ammonia and
chlorine)
Dechlorination
DW03781
Source: Site Visit Reports.
Conventional filtration includes coagulation/flocculation, sedimentation, and filtration processes.
Direct filtration includes coagulation/flocculation and filtration processes.
DCN – Document control number (for project record).
2-5

Drinking Water Industry Report Section 2 – Data Sources
2.2.2 Description of Questionnaire
In February 2007, EPA mailed the Water Treatment Plant Questionnaire to 616
CWSs. EPA designed the survey to collect system- and plant-specific information. The survey
included three parts: 1) the first part identified the system and asked screening questions to
determine if the remainder of the survey should be completed; 2) the second part requested
information on WTPs operated by the CWS that generate residuals and serve more than 10,000
people; and 3) the third part requested financial data about the system.
EPA excluded small systems (serving less than 10,000 people) from the survey
mailing list. Even though there are a large number of small systems—over 48,000 small CWSs
(U.S. EPA, 2008), EPA estimated that these systems contribute a small percent of residuals
generated and discharged by the industry. In its supporting statement to the ICR, EPA estimated
that CWSs that serve less than 50,000 people would contribute less than nine percent of the
residuals from the industry.
The first part of the survey (question 1) requested system information (system
name, address, and contact information) and asked questions to determine if the system was
included in the scope of the questionnaire. A system was considered in scope if it was classified
as a community water system and if one or more of the WTPs operated by the CWS met two
criteria: 1) generated residuals in 2006; and 2) served a population greater than 10,000 people.
Because the CWS could operate more than one WTP, EPA only wanted to collect data on the
larger WTPs that generated residuals. If the respondent answered “no” to any of the questions,
the respondent was not required to proceed with completion of the survey. This part also asked
whether the system conducted or participated in any monitoring or other studies to assess
potential impacts from discharges of residuals.
The second part of the survey (questions 2 and 3) requested general treatment
plant information, production data, and current residuals treatment and disposal practices:
• Plant address;
• Population served;
2-6

Drinking Water Industry Report Section 2 – Data Sources
• Annual production;
• Age of plant and any current upgrades;
• Source water types (i.e., ground water, surface water, or purchased water);
• Source water treatment;
• Treatment chemicals used;
• Types and quantities of residuals generated, along with any treatment or
disposal practices;
• Pollution prevention practices;
• Discharge information; and
• NPDES permit and 2004 through 2006 DMR data for direct dischargers.
EPA used the collected data to develop a profile of the industry and to evaluate
relationships between production factors (e.g., population served, source water treatment
operations) and residuals quantity, characteristics, and waste management practices. The Agency
also used data received in response to these questions to identify treatment technologies in place
and zero discharge practices.
The last part of the survey (questions 4 through 13) requested financial data on the
parent utility. Survey questions included production data, population served, and water sales
revenue; drinking water systems that purchase water from that utility; other revenue sources;
total revenue; residential customers and sales revenue from 2004 to 2006; residential customer
zip codes; billing structure; programs to lower cost for low- or fixed-income households;
expenses; and cost for capital improvements, repairs, or expansions. EPA used this information
to characterize the economic profile of the industry.
2.2.3 Development of the Survey Mailing List
The questionnaire focused on CWSs that operate treatment plants that serve more
than 10,000 people (estimated based on system population served and corresponding plant
production) and generate residuals. To develop the list of potential survey recipients, EPA
2-7

Drinking Water Industry Report Section 2 – Data Sources
identified CWSs that serve more than 10,000 people using EPA’s Safe Drinking Water
Information System (SDWIS) database from November 9, 2006 (U.S. EPA, 2006). In addition,
EPA identified wholesale systems in SDWIS (e.g., list service population of 25) and determined
the systems’ downstream population served by reviewing EPA’s 2000 Community Water System
Survey (U.S. EPA, 2002) and system websites (ERG, 2005). If a wholesale system served a
downstream population exceeding 10,000 people, EPA included that system in its survey mailing
list. EPA identified 4,115 CWSs that serve more than 10,000 people.
EPA then identified whether these systems operated WTPs that potentially
generated residuals. To identify treatment operations, EPA used data from SDWIS, the 2000
Community Water System Survey, Internet searches, and the OGWDW Information Collection
Rule Auxiliary 1 database.
1
EPA excluded systems with plants that perform only disinfection or
chemical addition as these plants do not generate residuals. EPA’s final list of potential survey
recipients included 2,290 CWSs. EPA used the mailing addresses listed in SDWIS. For more
information about SDWIS and other OGWDW data sources, see Section 2.3.
2.2.4 Sample Selection
EPA focused its analysis on the characteristics of large systems serving more than
50,000 people and those that primarily use surface water because these systems (and their WTPs)
are expected to discharge the majority of the WTP residuals, i.e., pollutant loadings.
Consequently, EPA sampled a greater percentage of systems serving more than 50,000 people
and surface water systems than systems serving 10,001 to 50,000 people and ground water
systems. Appendix A provides information on how the Agency designed the survey, developed
the sample size, and extrapolated the survey results.
2.2.5 Survey Response
EPA mailed 616 electronic surveys, and received 552 responses for a 90 percent
response rate. Of the 552 responses, 482 were in scope based on responses to Questions 1c to 1e,
1
Data collected by the Information Collection Rule (U.S. EPA, 2000) pertains to the Safe Drinking Water Act and
differs from EPA’s Information Collection Request performed as part of this industry review.
2-8

Drinking Water Industry Report Section 2 – Data Sources
on generation of residuals in 2006, operation of one or more WTPs serving more than 10,000
people, and classification as community water system.
As part of its technical analysis, EPA developed a survey review checklist to
determine whether the responses received for the second part of the survey (questions 2 and 3)
were complete. If survey responses were not complete or unclear, EPA contacted the system or
WTP representative for clarification.
Follow-up included review of responses and personal communication with system
contacts if survey responses were incomplete or if there were questions concerning the data
reported. Based on the survey review and follow-up communication, EPA incorporated changes
to the survey response to the extent possible. EPA either updated the electronic survey database
submitted by the CWS or marked a hard copy of the survey submittal prior to data entry into a
database. All in-scope and complete responses were combined into a single survey response
database. EPA determined that 378 of the in-scope technical survey responses were complete and
included those responses in the survey response database – technical data (U.S. EPA, 2009).
As part of its economic analysis, EPA reviewed the third part of the survey
(questions 4 through 13). These questions allowed respondents to provide information for the
parent utility (i.e., representing multiple systems). EPA included economic data for 482 systems
in the survey response database – financial data (U.S. EPA, 2010). Not all the DWT systems
included in the survey response database – financial data were included in the database with
technical responses. For the DWT systems not included in the survey response database –
technical data, EPA reviewed a subset of the technical responses to determine the types and sizes
of the systems. These data were used for the national estimates (see Appendix A).
2.2.6 Protection of Confidential Business Information
EPA recognizes that certain data submitted by the industry has been claimed as
confidential business information (CBI). The Agency has withheld CBI from this report,
including aggregate data that represents a small number of systems or WTPs. The Agency’s
2-9

Drinking Water Industry Report Section 2 – Data Sources
approach to CBI protection ensures that data made available to the public explain the industry
review without compromising data confidentiality.
2.3 EPA’S GROUND WATER AND DRINKING WATER DATA
EPA, along with delegated states and tribes, implements the requirements of the
Safe Drinking Water Act, which safeguards drinking water delivered to consumers’ taps. EPA
regulates 90 percent of the public drinking water supply in the United States. Public water is
supplied by publicly- or privately-owned systems that serve at least 25 people or at least 15
service connections for 60 days or more per year. EPA does not regulate private water supplies
that serve one or a few homes, such as household wells (U.S. EPA, 2003).
EPA maintains the SDWIS database (Section 2.3.1); collects system- and plant-
level data from the industry (Sections 2.3.2 and 2.3.3); and provides other data on the industry
(Section 2.3.4). EPA used these data to identify systems that serve more than 10,000 people,
including system and treatment plant characteristics. EPA created the survey mailing list for the
industry questionnaire using these data.
2.3.1 Safe Drinking Water Information System
EPA maintains basic information about the nation’s drinking water supply in
SDWIS
2
. States and EPA regional offices report data to EPA quarterly on all public water
systems. Each public water system is identified in SDWIS using a nine character identification
number, which includes the identification of the state or EPA regional office that oversees the
system’s compliance. Data reported include basic information on the systems such as the
following:
• System name and address;
• Retail population served;
• Number of service connections;
2
U.S. EPA maintains SDWIS/Federal database which is described in Section 2.3.1. In addition to the federal
database, SDWIS/State is maintained by the drinking water primacy agency (e.g., state) and may contain additional
data not available in the federal database.
2-10

Drinking Water Industry Report Section 2 – Data Sources
• Primary county or city served;
• Type of system (i.e., CWS or other);
• Ownership;
• Primary source water type (ground water or surface water); and
• Enforcement data.
SDWIS includes both mandatory and optional reporting components. Optional
reporting components include ownership and type of treatment. Because providing some data is
discretionary, EPA does not have complete data on every system for these parameters. If
treatment is included in SDWIS, the data are on a plant-specific basis and include treatment
objectives such as the following:
• Corrosion control;
• Dechlorination;
• Disinfection;
• Disinfection by-products control;
• Inorganics removal;
• Iron removal;
• Manganese removal;
• Organics removal;
• Particulate removal;
• Radionuclides removal;
• Taste/odor control;
• Softening (hardness removal); and
• Other.
SDWIS does not include data on the type and quantity of residuals generated,
residuals treatment method, or residuals disposal method. Therefore, EPA gathered data on
residuals generation, treatment, and disposal using the industry questionnaire (see Section 2.2).
SDWIS is continually updated, but EPA maintains snapshots (or freezes) of the
database. In 2006, there were 156,644 public drinking water systems (U.S. EPA, 2008):
• 52,339 community water systems (i.e., systems that supply water to the
same population throughout the year) serving 282 million people.
• 19,045 non-transient, non-community water systems (i.e., systems that
regularly supply water to at least 25 of the same people for six months or
more per year, such as schools) serving 6 million people.
2-11

Drinking Water Industry Report Section 2 – Data Sources
• 85,260 transient, non-community water systems (i.e., systems that supply
water at locations where people do not remain for an extended time
period, such as a campground) serving 14 million people.
2.3.2 2000 Community Water System Survey
To support the development and evaluation of drinking water regulations, EPA
collected industry data in the 2000 Community Water System Survey (CWSS). EPA collected
operational and financial characteristics in the CWSS. Because CWSs are a very diverse group,
CWSS is stratified to represent the complete population of CWSs across the United States, based
on a list of approximately 52,000 systems from SDWIS. For the 2000 CWSS, questionnaires
were mailed to 1,200 medium and large systems, and 600 site visits to small systems (serving
3,300 people or fewer) were conducted.
3
Operational data requested include the following:
• System ownership type;
• Source water type (ground water, surface water, or purchased water) and
description of source;
• Raw water concentrations;
• Production quantity, flow rate, and capacity for plants;
• Type of source water treatment;
• Filter backwash technique;
• Residuals treatment and management;
• Discharge type;
• Operator information; and
• Storage and distribution information.
3
Site visits were used instead of mailed questionnaires from small CWSs to reduce the burden of the information
collection effort on small systems.
2-12

Drinking Water Industry Report Section 2 – Data Sources
Financial characteristics collected include customer type, revenue, billing structure, expenses,
and source of funds. The overall response rate was 69 percent. Responses from CWSS were then
weighted to develop estimates from the CWS community as a whole.
The 2000 CWSS data included a report, an MS Access® database of the survey
information, and an MS Excel® spreadsheet containing treatment plant-specific data. The 2000
CWSS includes data on 2,603 WTPs at 1,246 systems. EPA used information from the 2000
CWSS to assist in sample frame development and to characterize the economic profile of the
industry.
2.3.3 Information Collection Rule
The purpose of the EPA Information Collection Rule, 40 CFR Part 141 (May 14,
1996), was to generate and provide EPA with the following information from drinking water
systems:
• Monitoring data on microbiological contaminants;
• Monitoring data on disinfection by-products;
• General water quality data; and
• Treatment plant design and operating information to characterize the
system.
EPA collected these data from drinking water systems and analytical laboratories
and entered them in the Information Collection Rule Federal Database. To facilitate review of
the data, EPA designed seven auxiliary databases to store subsets of data extracted from the
Information Collection Rule Federal Database. EPA used data from the Information Collection
Rule Auxiliary 1 Database (U.S. EPA, 2000), along with supporting documentation, to
characterize systems and treatment plants in the DWT industry as part of the survey mailing list
development. The Auxiliary 1 Database includes information for 296 systems (all but nine
systems serve populations greater than 50,000 people).
Data available in the Auxiliary 1 Database include the following:
2-13

Drinking Water Industry Report Section 2 – Data Sources
• System design (e.g., EPA region, storage volume, distribution time,
number of booster stations and dose range);
• System monitoring (e.g., population served, average flow rate—wholesale
and retail);
• Wholesale purchase flow rate;
• Treatment plant design (e.g., treatment process, average percent solid,
solid handling capacity, clearwell data, and minimum temperature);
• Plant monitoring data (e.g., alum dose (parts per million, ppm), iron dose
(ppm), coagulant type, source water type (surface water or ground water),
sludge production, sludge percent solids, disinfection type, average
influent flow rate, sampling event influent flow rate, chlorine (Cl
2
)
demand, effluent flow rate (average and sample event), wastewater
residuals treatment performed, wastewater treatment flow rate (average
and sample event));
• Unit process data (e.g., sequence in treatment train, volume, filtration
surface area, residence times, process flow rate, filtration media type and
depth, granular activated carbon (GAC) depth, disinfectant name and dose
(ppm));
• Chemical feed information (e.g., alum, iron, Cl
2
);
• Ozone chamber data;
• Sampling data;
• Water quality monitoring data; and
• Intake information (e.g., latitude and longitude, reach).
2.3.4 Other Ground Water and Drinking Water Data
EPA staff and the EPA website provided additional information to support this
industry review. The EPA website includes the following information:
• Basic drinking water treatment references;
• Drinking water regulations and standards;
2-14

Drinking Water Industry Report Section 2 – Data Sources
• List of drinking water contaminants and maximum contaminant levels
(MCLs) allowed in drinking water;
• Guidance on drinking water regulations and standards; and
• Additional data on the Safe Drinking Water Act.
2.4 OTHER INFORMATION COLLECTION ACTIVITIES
EPA completed other data collection efforts to supplement information gathered
through the aforementioned site visits, surveys, and EPA data sources, the purpose of which was
to obtain information on the documented environmental impacts of discharges from WTPs, water
treatment operations, residuals characteristics, pollution prevention practices, residuals treatment
technology innovation, and best management practices. These other data collection activities
included a review of literature sources, current NPDES permits, NPDES monitoring reports,
other EPA data sources, industry data (on-line data from drinking water system web pages), and
AWWA surveys and reports.
2.4.1 Literature Search
EPA conducted a literature search to obtain information on various aspects of the
DWT industry. EPA performed several Internet and literature searches to identify papers,
presentations, and other applicable materials. Literature collected by EPA covers such topics as:
• Source water treatment technologies;
• Water quality and treatment;
• Pollution prevention;
• Characterization of WTP residuals;
• Residuals treatment, including performance and costs;
• Disposal practices and waste management of residuals (e.g., sludge,
concentrate streams);
• Recycling and reuse of waste streams;
2-15

Drinking Water Industry Report Section 2 – Data Sources
• Industry trends;
• Environmental impacts; and
• Effect of discharges on the environment.
EPA used data from these literature sources to develop the industry questionnaire,
identify and characterize residuals, determine applicability of pollution prevention techniques,
identify residuals treatment technologies, and identify best management practices (BMPs).
2.4.2 Current NPDES Permits
EPA collected available permit information to determine current practices in
setting discharge limits for WTPs. States and, in some cases, EPA regions, issue NPDES permits
to WTPs that allow direct discharge of wastewater. States might issue general permits for groups
of plants that have similar operations and wastewater characteristics. States issue individual
permits for specific plants that do not meet the requirements of the states’ general permits.
Section 4.1.7 provides an overview of NPDES permits. Depending on the permit requirements,
dischargers report compliance with NPDES permits via monthly discharge monitoring reports
(DMRs) submitted to the permitting authority.
EPA’s Permit Compliance System (PCS) database contains monitoring and
NPDES permit data from some permittees that discharge wastewater directly to surface waters.
States (or other permitting authority) have some discretion as to which data they make available
to PCS.
4
For example, permitting authorities enter DMR and permit information for facilities
that are considered major dischargers. However, they do not necessarily enter DMR or permit
information into PCS for minor dischargers (as opposed to major dischargers) or facilities
covered by a general permit.
Permitting authorities designate which facilities are considered major dischargers
or minor dischargers based on the likelihood that the discharge will impact receiving waters if
4
EPA used DMR data from 2005, when DMR data were still maintained solely in PCS. Starting in 2006, states
began reporting their data to the Integrated Compliance Information System for NPDES (ICIS-NPDES). However,
this system was not in use at the time of this study.
2-16

Drinking Water Industry Report Section 2 – Data Sources
not controlled. Facilities designated as major dischargers must submit monthly DMRs to the
permitting authority, who enters the reported DMR data into PCS. States have the option to enter
DMR data for minor discharges into PCS, however, EPA does not require states to enter the data.
For this reason, the permitting authority may choose to include data only for a limited set of
minor dischargers in PCS. Similarly, EPA does not require DMRs for facilities covered under
general permits, and PCS may include limited or no data on general permits.
Therefore, the completeness of the data in the PCS system is much higher for
larger facilities that are more likely to impact surface waters. Information on smaller facilities
with less likelihood to impact surface waters is not consistently tracked in PCS. Also,
information may not be available for facilities with discharges covered under a general permit.
Despite the expected data limitations, EPA extracted available information from
PCS to identify WTPs with NPDES permits. The extraction was performed by searching PCS
using the Standard Industrial Classification (SIC) code 4941 for the drinking water treatment and
supply industry. EPA found that PCS contains information on approximately 3,000 WTPs with
NPDES permits; however only 20 plants are major dischargers. As a result, only limited data
were available on WTP NPDES permits in PCS. EPA used this information as part of its initial
screening process to determine the number of plants that discharge directly to waters of the
United States.
EPA expanded its search for WTP permit information beyond PCS, obtaining
permits available online and those collected by other EPA activities (i.e., site visits and surveys).
EPA used these permits to study permit requirements and treatment in place at WTPs that had
certain water treatment operations. The majority of the limits in NPDES permits for WTPs were
based on best professional judgment (BPJ). EPA summarized the current permit discharge
requirements based on best professional judgment (BPJ)-based permit limitations (see Section 5).
2.4.3 NPDES Discharge Monitoring Reports (DMRs)
NPDES-permitted plants submit DMRs to their permitting authority (state or EPA
Region). DMRs summarize the quality and volume of wastewater discharged from plants with
2-17

Drinking Water Industry Report Section 2 – Data Sources
NPDES permits. They are critical for determining compliance with NPDES permit provisions for
reporting and monitoring and for generating national trends in Clean Water Act compliance.
DMRs may be submitted monthly, quarterly, or annually depending on the requirements of the
NPDES permit.
EPA requested DMR data (for years 2004, 2005, and 2006) as part of the 2007
industry survey. EPA received primarily 2006 DMR data. EPA used the DMR data to identify
pollutants of concern (pollutants currently included in NPDES permits) and to calculate pollutant
loading estimates. EPA received 2006 DMR data for 140 WTPs (U.S. EPA, 2007).
Indirect dischargers file compliance monitoring reports with their control
authority (e.g., POTW) at least twice a year as required under the General Pretreatment
Standards (40 CFR Part 403), while direct dischargers file DMRs with their permitting authority
at least once a year. EPA did not collect compliance monitoring reports for WTPs that are
indirect dischargers. This information is less centralized and therefore more difficult to collect
than information on direct dischargers.
2.4.4 Other EPA Data
EPA reviewed two additional databases, the Facility Registry System (FRS) and
the Resource Conservation and Recovery Act (RCRA) database, to gather additional data on the
DWT industry. These databases classify facilities using a four-digit Standard Industrial
Classification (SIC) code or five-digit North American Industry Classification System (NAICS)
code. EPA used SIC code 4941 or NAICS code 22131 (Drinking Water Treatment and Supply
Industry) to search the databases.
2.4.4.1 Facility Registry System (FRS)
The FRS is a centrally managed database that identifies facilities, sites, or places
subject to environmental regulations or of environmental interest. This database links the various
identification numbers from federal and state environmental programs for a single facility. At the
time of EPA’s review of the FRS data, the public water system identification numbers from
2-18

Drinking Water Industry Report Section 2 – Data Sources
SDWIS were not all matched to FRS identification numbers. The matching is complicated by the
fact that a water system (assigned a single Public Water System identification number, PWSID,
in SDWIS) may operate more than one plant subject to environmental regulations (e.g., multiple
NPDES permit IDs may apply to a single PWSID). The FRS database includes information for
over 8,000 plants in SIC code 4941. EPA matched FRS IDs (and corresponding NPDES permit
IDs) to specific WTPs (and their PWSID) where possible to assist in identifying direct
dischargers included in the survey mailing list.
2.4.4.2 Resource Conservation and Recovery Act (RCRA)
If a WTP generates solid waste, it may be subject to RCRA storage, treatment,
and disposal requirements. RCRA provides guidelines for the management of solid and
hazardous wastes. In order to be classified as hazardous, wastes must be listed under 40 CFR
Part 261 of RCRA. To be considered a RCRA hazardous waste, drinking water residuals must
either contain a constituent listed as a hazardous waste in RCRA, or exhibit certain
characteristics of ignitability, corrosivity, reactivity, or toxicity. Information that EPA collected
on the constituents of residuals indicates that the residuals could be considered RCRA hazardous
if they meet the criteria of toxicity or corrosivity. The FRS database lists 457 WTPs assigned a
RCRA identification number. RCRA waste management requirements, and any associated costs,
may be part of the review process when developing BMPs or considering alternatives to effluent
discharges.
2.4.5 Industry Data
EPA used industry data, such as system websites and consumer confidence
reports, to supplement data on specific systems and their operations. For example, EPA
identified wholesale systems (i.e., those that sell drinking water to other systems but do not
distribute to retail customers) serving more than 10,000 people by reviewing system websites.
EPA also used on-line data to gather information on a plant-specific level, such as treatment
performed and source water type. These industry data filled data gaps or confirmed data
provided by other data sources (e.g., OGWDW data sources).
2-19

Drinking Water Industry Report Section 2 – Data Sources
2.4.6 American Water Works Association (AWWA) Surveys and Reports
The AWWA trade association represents water treatment systems and service
providers (e.g., consultants, manufacturers of water treatment products, etc.), as well as
individual members who are most often professionals in the drinking water industry. AWWA
provides regulatory support, technology updates, and other services to its members. EPA
reviewed reports and other data available from AWWA. A summary of the AWWA surveys and
resulting reports is provided below.
2.4.6.1 2004 Water and Wastewater Rate Survey
The 2004 Water and Wastewater Rate Survey includes summary data and system-
specific data for water and wastewater systems. Data from the survey include the following:
• Rate trends;
• Rates by geographic area;
• Utility characteristics (e.g., population served, daily gallons sold, daily
capacity, maximum daily production, number of employees, and financial
data, including annual capital needs, total assets, long-term debt, and total
equity);
• Rate structure, monthly water charges, other water charges (e.g., minimum
monthly charge for residential and industrial, connection charge, system
development charge), total revenues, and total operating expenses;
• Indication of whether utilities provide water outside the municipal or
district boundaries (e.g., wholesale) and retail differential (i.e., how much
more “outside” customers pay compared to “inside” customers); and
• Median household affordability index.
Survey participants include 266 water treatment utilities from 50 states and six
Canadian provinces. For comparison, the survey contains international utility data from 44 cities
in 27 countries (AWWA, 2004). EPA reviewed the survey results for background data on the
industry; however, EPA did not make additional use of the survey results for this report.
2-20

Drinking Water Industry Report Section 2 – Data Sources
2.4.6.2 2002 AWWA Recycle Survey Analysis
AWWA surveyed WTPs to determine their recycling practices for spent filter
backwash water and other waste streams. AWWA compiled and analyzed data from 333 plants
that responded to the survey and indicated recycling of one or more streams. The survey gathered
data on the following:
• Size of treatment plant (capacity and population served);
• Location (state);
• Source water type and its treatment;
• Percent recycled backwash;
• Treatment performed on waste stream prior to recycling back into plant;
• Point where recycled stream reenters the source water treatment;
• Discharge permit availability for the waste stream; and
• Indication of whether monitoring data on the waste stream are available.
The analysis included determination of whether each plant’s equalization basin
was adequately sized for the recycle stream and whether each plant’s sedimentation basin was
adequately sized to serve as the equalization basin for the recycle stream (AWWA, 2002). EPA
reviewed the survey results for background data on the industry; however, EPA did not make
additional use of the survey results for this report.
2.4.6.3 Residuals Management Costing Analysis
To evaluate the cost considerations to construct and operate residuals treatment
systems, EPA reviewed an AWWA-sponsored report entitled Costing Analysis to Support
National Drinking Water Treatment Plant Residuals Management Regulatory Options, dated
April 2008 (AWWA, 2008). In this report, AWWA estimated costs to install and operate a
typical sludge treatment system at model plants. AWWA developed cost curves for conventional
filtration plants and lime softening plants, over a range of flows and solids loadings. AWWA
presented their results as a series of curves, showing cost relative to population served, by plant
type and solids loading. EPA used the costing analysis to augment its summary of cost
considerations for residuals treatment (see Section 13).
2-21

Drinking Water Industry Report Section 2 – Data Sources
2.5 STAKEHOLDER MEETINGS
From 2004 through 2008, EPA participated in several meetings with other EPA
offices, permitting authorities, industry representatives, industry associations, technology
vendors, and other interested parties to gather technical information on environmental and
operational issues related to drinking water treatment and supply operations. The purpose of the
meetings was to gather current detailed information about the industry. These meetings also
served as forums for the transfer of information between EPA and industry representatives on all
aspects of WTP operations.
EPA participated in meetings with the following groups:
• EPA offices: OGWDW, Office of Enforcement and Compliance
Assurance (OECA), Office of Research and Development (ORD), and
Office of Pollution Prevention and Toxics (OPPT).
• Permit contacts from EPA Regions 1 through 10 and the following states
and territories: Arkansas, Colorado, Florida, Maryland, Puerto Rico,
Texas, and Virginia.
• Trade associations and industry representatives:
— American Water Works Association,
— Association of Metropolitan Water Agencies,
— American Membrane Technology Association,
— Water and Wastewater Equipment Manufacturer Association,
— Wateruse,
— Passaic Valley Water Commission,
— National Association of Clean Water Agencies,
— Greater Cincinnati Water Works,
— East Bay Municipal Utility District, and
— Los Angeles Department of Water and Power.
• Drinking water treatment technology vendors and/or consultants:
— F.B. Leopold Company,
— US Filter,
— General Electric, and
— Black & Veatch.
• Other interested parties:
— Natural Resources Defense Council.
2-22

Drinking Water Industry Report Section 2 – Data Sources
In addition to the meetings, EPA also attended several AWWA conferences
including the following:
• AWWA Water Quality Technology Conference, November 2004;
• AWWA Annual Meeting and Conference, June 2005;
• Water Environment Technical Exhibit and Conference, October 2005; and
• AWWA Annual Meeting and Conference, June 2006.
By participating in these meetings and conferences, EPA was able to obtain up-to-
date information about source water treatment methods; residuals generation, collection,
treatment, and disposal practices; and economic and financial aspects of the industry. EPA used
this information throughout its industry review.
2.6 DRINKING WATER TREATMENT TECHNOLOGY REVIEW
As part of the industry review, EPA solicited early individual input from
stakeholders on technical issues related to the management of drinking water residuals. Goals for
this stakeholder review included the following:
• Characterization of typical residuals;
• Identification of pollutants of concern;
• Identification of pollution prevention and treatment technologies for
residuals;
• Evaluation of 1993 and 1987 cost estimates developed by EPA and
AWWA, respectively, for these residuals treatment technologies (U.S.
EPA, 1993; AWWA, 1987); and
• Application of prevention and treatment technologies.
From 2005 through 2007, EPA held several meetings and provided stakeholders
with various technical papers to review. EPA reviewed the comments received from stakeholders
and prepared technical paper comment-response documents.
Stakeholders included personnel from American Membrane Technology
Association, AMWA, Association of State Drinking Water Administrators (ASDWA), AWWA,
2-23

Drinking Water Industry Report Section 2 – Data Sources
Black & Veatch, CH2M Hill, EE&T, East Bay Municipal Utility District, Carollo Engineers,
P.C., Cincinnati Water Works, City of St. Louis Water Division, Environmental Law and Policy
Center of the Midwest, F.B. Leopold Co., Los Angeles Department of Water and Power,
NACWA, US Filter, and Water Environment Research Federation, as well as EPA’s OGWDW
and ORD.
2.7 REFERENCES
American Water Works Association (AWWA), 1987. Water Treatment Plant Waste
Management. Document Control Number (DCN) DW00186.
AWWA, 2002. 2002 Recycle Survey Analysis. Submitted by Environmental Engineering &
Technology, Inc. for AWWA. Document Control Number (DCN) DW00926.
AWWA, 2004. AWWA and Raftelis Financial Consultants, Inc, 2004 Water and Wastewater
Rate Survey. DCN DW03765.
AWWA, 2008. Costing Analysis to Support National Drinking Water Treatment Plant Residuals
Management Regulatory Options, Submitted by Environmental Engineering & Technology, Inc.,
Newport News, VA. DCN DW03766.
Eastern Research Group (ERG), 2005. Memorandum: Review of Wholesale Drinking Water
Treatment Systems, Chantilly, VA. August 1, 2005. DCN DW03783.
U.S. Environmental Protection Agency (EPA), 1993. Large Water System Byproducts Treatment
and Disposal Cost Document (EPA 811-D-93-002), Office of Water, Washington, DC. DCN
DW00058.
U.S. EPA, 2000. Information Collection Rule (ICR) Auxiliary 1 Database. Office of Water,
Washington, DC. DCN DW03723.
U.S. EPA, 2002. Community Water System Survey 2000 (EPA 815-R-02-005), Office of Water,
Washington, DC. DCN DW00001.
U.S. EPA, 2003. Water on Tap: What You Need to Know (EPA 816-K-03-007), Office of Water,
Washington, DC. DCN DW00653.
U.S. EPA, 2006a. SDWIS Inventory 2006-11-09 (MS Excel® file), Office of Water,
November 9, 2006. DCN DW03717.
U.S. EPA, 2006b. Survey Sample Frame Version 4. Office of Water, Washington, DC,
December 2006. DCN DW03716.
2-24

Drinking Water Industry Report Section 2 – Data Sources
U.S. EPA, 2007. Phase I DMR Database. Office of Water, Washington, DC, December 2007.
DCN DW03703.
U.S. EPA, 2008. FACTOIDS: Drinking Water and Ground Water Statistics for 2006 (EPA 816-
K-06-012). Office of Water, Washington, DC. DCN DW03755.
U.S. EPA, 2009. Drinking Water Survey Response Database – Technical Data for the 2006
Drinking Water Industry Questionnaire. Office of Water, Washington, DC. DCN DW03790.
U.S. EPA, 2010. Drinking Water Survey Response Database – Financial Data for the 2006
Drinking Water Industry Questionnaire. Office of Water, Washington, DC. DCN DW03789.
2-25

SECTION 3
INDUSTRY PROFILE
The purpose of the drinking water treatment (DWT) industry is to provide potable
water to its customers. The DWT industry falls under Standard Industrial Classification (SIC)
code 4941, which crosswalks with North American Industry Classification System (NAICS)
code 22131. In addition to drinking water, SIC code 4941 includes other water supply plants—
those that treat water for use in commercial and industrial applications. NAICS code 22131
includes all of SIC code 4941 plus irrigation systems (defined by SIC code 4971). For this
industry review, EPA focused on drinking water systems that serve more than 10,000 people.
Most systems that serve more than 10,000 people are defined as community water systems
(CWSs) under the Safe Drinking Water Act. CWSs serve the same customer base year round
(e.g., city water authority).
Drinking water systems may obtain their water supply either directly from the
source (e.g., river, lake, reservoir for surface water sources or via wells for ground water sources)
or may purchase from wholesalers. Systems may treat the source water (i.e., intake water) prior
to distribution or only provide delivery of the drinking water. If the system treats the source
water prior to delivery, the system operates one or more water treatment plants (WTPs).
3.1 OVERVIEW OF DWT INDUSTRY
As discussed in Section 2.3.1, there are 52,339 community water systems (CWSs)
in the United States. EPA determined that 4,115 CWSs serve more than 10,000 people.
5
EPA’s
industry questionnaire collected data on CWSs that operate large WTPs (i.e., plants that produce
drinking water for more than 10,000 people). Of the 4,115 CWSs, 42 percent (1,742 CWS)
operated large WTPs that generate residuals (e.g., wastewater, slurry). See Appendix A. The
other 58 percent of CWSs either operate only small WTPs (produce drinking water for less than
5
2006 data from EPA’s Safe Drinking Water Information System and on-line review of wholesale systems (U.S.
EPA, 2006; ERG, 2005).
3-1

Drinking Water Industry Report Section 3 – Industry Profile
10,000 people) or do not generate residuals at the large WTPs (e.g., perform disinfection of the
source water only).
WTPs may dispose of residuals by discharging into waters of the United States
(direct discharge) or by discharging via sewer to a publicly-owned treatment works (indirect
discharge). Of the WTPs serving more than 10,000 people and generating residuals, EPA
estimates that 31 percent are direct dischargers, 37 percent are indirect dischargers, and 7 percent
are both direct and indirect dischargers. The Agency estimates that the remaining 25 percent of
WTPs are zero dischargers (i.e., do not discharge directly or indirectly). Zero discharge methods
include recycling, evaporation, composting, landfill disposal, spray irrigation, underground
injection, and land application. Table 3-1 summarizes the number of WTPs operated by CWSs
by source water treatment method and discharge status (see Appendix A).
Table 3-1. Discharge Status for Water Treatment Plants Serving More than 10,000 People
Size of CWS
Population
Served
Primary
Water
Source
Source Water
Treatment Method
Total
Number
of WTPs
a
Estimated Number of WTPs by Discharge Type
Direct
Discharge
a
Indirect
Discharge
a
Zero Discharge
Only
b
10,001 –
50,000
Ground
Any
526
121
307
107
Surface
Any
938
406
405
206
More than
50,000
Ground
Precipitative softening
66
31
2
32
Conventional filtration
14
[CBI Redacted]
[CBI Redacted]
[CBI Redacted]
Membrane
c
19
2
6
13
Ion exchange
8
[CBI Redacted]
[CBI Redacted]
[CBI Redacted]
Other treatment
d
4
[CBI Redacted]
[CBI Redacted]
[CBI Redacted]
Surface
Precipitative softening
168
90
55
40
Conventional filtration
383
167
142
123
Membrane
c
19
4
12
2
Ion exchange
0
0
0
0
Other treatment
d
6
[CBI Redacted]
[CBI Redacted]
[CBI Redacted]
Total
2,151
a
832
a
943
a
531
Source: Appendix A.
a – WTPs may handle residuals using multiple methods; therefore, totals for each column exceed the total number of plants (e.g.,
155 WTPs discharge both directly and indirectly).
b – Zero discharge methods include recycling, evaporation, composting, landfill disposal, spray irrigation, underground injection,
and land application. Direct and indirect dischargers may also use these methods, however, those WTPs are not included in the
zero discharge only plant counts.
c – Membrane treatment method includes microfiltration, ultrafiltration, and membrane desalination (reverse osmosis,
nanofiltration, electrodialysis, and electrodialysis reversal).
d – Other treatment methods include filtration without coagulation and adsorption processes. This group also includes plants that
did not indicate any treatment operation in the survey (classified as “none”).
3-2

Drinking Water Industry Report Section 3 – Industry Profile
3.1.1 Types of Drinking Water Systems
Drinking water systems that provide water to at least 25 people or 15 service
connections are defined as “public water systems” (Section 1401(4)(a)). Public water systems
encompass a wide variety of systems and plants. In total, there are 155,693 active public water
systems serving 307 million people in the United States (U.S. EPA, 2007). These systems differ
in terms of the type of population that they serve (residential, non-residential, transient, or
permanent) and in terms of the entity that owns them (public, private, or a mixture of both).
EPA further defines public water systems into the following three types:
• Community water system (CWS): supplies water to the same population
year round.
• Non-transient, non-community water system (NTNCWS): regularly
supplies water to at least 25 of the same people six months or more per
year, but not year round (e.g., schools, factories, offices, and hospitals
with their own water system).
• Transient, non-community water system (TNCWS): supplies water in such
places as a gas stations or campgrounds where people do not remain for an
extended time period.
These are the drinking water systems usually associated with tap water.
Households outside the service area of a water system obtain drinking water from private wells.
3.1.2 How EPA Classifies Drinking Water Systems
EPA classifies the size of a drinking water system by population served (size),
ownership type, and source water type. Other measurements for classifying the size include
finished water production volume and number of employees. The population served often
corresponds to the production volume and number of employees needed to run the system. The
majority of the drinking water systems serve 10,000 people or less; however, the majority of the
drinking water is produced by larger systems (those serving more than 10,000 people) (U.S.
EPA, 2008a).
3-3

Drinking Water Industry Report Section 3 – Industry Profile
3.2 SUMMARY OF QUESTIONNAIRE RESPONSES
This section summarizes the responses to the industry questionnaire (i.e., survey)
about WTP and system conditions in 2006. Because EPA used statistical procedures to select
systems for the survey to be representative, the responses can be used to derive statistical
estimates for all systems and WTPs in the target population
6
. For this survey, the target
population is defined as all systems that operate WTPs that have the capability to generate (and
potentially treat) residuals and serve populations greater than 10,000 people. In addition, the
systems must be community water systems (CWSs).
The following subsections present a series of tables with the results of the
statistical analysis of the survey data. Each table presents national estimates based upon
responses from systems and WTPs statistically selected for the questionnaire. Section 3.2.1
describes the classification
7
of systems and WTPs by population served, source water, and
treatment type. Section 3.2.2 summarizes WTP characteristics reported in responses to questions
2 and 3 of the survey. Section 3.2.3 summarizes the system characteristics reported in response
to economic and financial questions 4 through 13. Appendix A describes the sample design, the
selection procedure, response rates, and the development of the national estimates.
3.2.1 System and WTP Classification
EPA used the responses to classify the WTPs by the size of the population served,
primary water source, and the source water treatment method. Systems, however, sometimes
have WTPs assigned to different classifications. For example, the system may operate two small
WTPs and one larger WTP. In another example, it may operate WTPs using different treatment
technologies. To assign each system into a single classification, EPA used the information
associated with the largest WTP reported in its response. Thus, each system is classified by the
population served, primary water source, and treatment method of its largest WTP. EPA
estimates that there are 2,151 WTPs in 1,742 systems in the target population.
6
The target population for a data system is the specific population about which information is desired.
7
As explained in Appendix A, classifications are “domains” in statistical nomenclature.
3-4

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-2 provides a summary of the number of WTPs and systems in each
classification. Because ion exchange operations were not reported for larger WTPs (i.e., serving
populations more than 50,000) using surface water, Table 3-2 shows the estimated number of
WTPs and systems to be zero. Because EPA does not have any data on such WTPs or systems,
the classification has been excluded from all other tables presented in this section.
To determine size classifications for Table 3-2 and the other tables in this section,
EPA used the response to question 2b (shown below in Figure 3-1) about the number of people
served by the WTP.
Figure 3-1. Question 2b: Population Served by the WTP in 2006
To assign each WTP to a single water source, EPA used the response to question
2e that asks about the percentages of water used from different sources (see Figure 3-2). Most
WTPs reported the majority of the water was either surface or ground water. For the seven WTPs
that only reported purchased water, EPA assigned them to surface water as their source because
it was the most likely source to require treatment after purchase. For two WTPs reporting other
water sources and one WTP with an even ground water/surface water allocation, EPA used the
most commonly reported source water in their size category.
Figure 3-2. Question 2e: Source Water Type
2.b. Please indicate the number people served by the water treatment plant in 2006.
Report your estimate to the nearest thousand (e.g., round 21,854 people served to
22,000). If you do not have this data readily available, see the instructions and example in
Question 1.d on page 2 to learn how to estimate the population served by your water
treatment plant.
_ _ _, _ _ _, 0 0 0 people
2.e. Please describe the type(s) of water used as the drinking water source in 2006.
Type of Source Water
Percentage of Total Source Water
Surface Water
Ground Water
Purchased Water
Other (specify):________________
Total
100%
3-5

Drinking Water Industry Report Section 3 – Industry Profile
In assigning WTPs to treatment methods, EPA evaluated their responses to
treatment and the types of chemicals reported in question 2.f of the survey.
Table 3-2. Industry National Estimates: Numbers of WTPs and Systems
Classification
Estimated Number of:
Size of Population
Served
Primary Water
Source
Treatment Method
Systems
WTPs
10,001-50,000
Ground
Any
378
526
Surface
Any
811
938
Subtotal
1,189
1,464
More than 50,000
Ground
Conventional Filtration
8
14
Membrane
19
19
Other
2
2
Softening
57
66
Ion Exchange
8
8
None
2
2
Subtotal
97
111
Surface
Conventional Filtration
295
383
Membrane
17
19
Other
4
4
Softening
139
168
Ion Exchange
0
0
None
2
2
Subtotal
456
576
Subtotal
554
688
Total
1,742
2,151
3.2.2 WTP Characteristics (Summary of Responses to Technical Questions)
This section provides national estimates based upon the responses to questions 2
and 3 that addressed WTP operations. The following information is summarized in each section:
• Section 3.2.2.1: basic WTP operating characteristics (Questions 2b – 2d);
• Section 3.2.2.2: source water treatment operations (Question 2f);
• Section 3.2.2.3: residuals treatment and pollution prevention practices
(Questions 2h and 2i);
3-6

Drinking Water Industry Report Section 3 – Industry Profile
• Section 3.2.2.4: residuals discharge practices (Question 2k); and
• Section 3.2.2.5: copper usage (Question 3).
The tables appear at the end of each subsection.
3.2.2.1 WTP Operating Characteristics (Question 2)
The survey collected basic operating characteristics data, including produced
water volume, number of people served, plant age, and water source in response to questions 2b,
2c, and 2d. Figure 3-3 shows the wording of the questions and the responses are summarized in
this section.
Table 3-3 presents the number of people served by WTP classification. Based
upon the responses, the target population served approximately 143 million people (i.e., 2,151
WTPs, each serving an average of 66,430 people).
Table 3-4 presents the total volume of finished water, the amount per person, and
water per day. The target population produced approximately 7.5 trillion gallons of finished
water per year (i.e., 2,151 WTPs, each producing an average of 3,490.2 million gallons per year).
Table 3-5 reports the minimum and maximum number of operating days based
upon the responses to question 2c. It also estimates the mean (average) number of days that the
WTP operated during the year. Most WTPs operate all or most of the year, although one WTP
reported only 12 days of operation. According to its website, it generally operates only when its
sister WTP is not operating.
8
Table 3-6 identifies when WTPs were built and most recently upgraded. The
oldest WTP in the survey was built in 1867, making it 140 years old in 2006 (i.e., year reported
in the survey). The median age of all WTPs was 36 years old (built in 1970). The WTP operating
8
Erie Water Works, http://www.eriewater.org/our-water/, retrieved December 15, 2008.
3-7

Drinking Water Industry Report Section 3 – Industry Profile
the longest since its last upgrade has been doing so since 1885 (121 years). The median time
since the last upgrade was 12 years.
Figure 3-3. Questions 2b-d: WTP Operating Characteristics
2.b. Please indicate the number people served by the water treatment plant in 2006.
Report your estimate to the nearest thousand (e.g., round 21,854 people served to
22,000). If you do not have this data readily available, see the instructions and example in
Question 1.d on page 2 to learn how to estimate the population served by your water
treatment plant.
_ _ _, _ _ _, 0 0 0 people
2.c. Please indicate the total amount of finished water produced at the water treatment
plant in 2006.
Report your estimate to the nearest million gallons (e.g., round 6,432,100 gallons
produced to 6,000,000).
_ _ _, _ _ _ , _ _ _ , 0 0 0, 0 0 0 gallons of finished water produced in
2006
Number of days in operation in 2006:
□ 365 days
□ _ _ _ days
2.d.i. Please indicate the year that this plant was first built (e.g., 1956).
_ _ _ _
Year
2.d.ii. Please indicate the year of the last treatment upgrade or significant expansion of
water treatment operations at this plant. A significant expansion is one that increases
capacity by 50% or more.
3-8

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-3. Number of People Served per WTP in 2006
(National Estimates Based on Responses to Question 2b)
Classification
Estimated
Number of
WTPs
Number of People Served (in thousands)
Size of
Population
Served
Primary
Water
Source
Treatment
Method
Minimum
Reported
Maximum
Reported
Estimated
Mean
Std Error
of Mean
10,001-
50,000
Ground
Any
526
10
50
18.86
1.72
Surface
Any
938
10
50
25.19
0.88
Subtotal
1464
10
50
22.92
0.95
More than
50,000
Ground
Conventional
Filtration
14
[CBI Redacted]
Membrane
19
59
132
79.37
5.53
Other
2
[CBI Redacted]
Softening
66
52
333
87
6.18
Ion Exchange
8
[CBI Redacted]
None
2
[CBI Redacted]
Subtotal
a
111
52
490
101.79
7.29
Surface
Conventional
Filtration
383
51
850
165.29
7.56
Membrane
19
56
104
82.14
5.52
Other
4
[CBI Redacted]
Softening
168
51
1,128
183.49
15.13
None
2
[CBI Redacted]
Subtotal
a
576
51
1,128
170.1
6.74
Subtotal
a
688
51
1,128
159.05
6.21
Total
a
2,151
10
1,128
66.43
2.89
a – CBI redacted counts of people served are included in subtotal and total rows.
3-9

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-4. Estimated Water Production per WTP in 2006 (National Estimates Based on Responses to Questions 2b and 2c)
Classification
Estimated
Number of
WTPs
Total Amount of Finished Water
(million gallons per year (MGY))
Estimated Water for
Each Person Served
Per Year
(gal/person/yr)
Estimated Water
Produced per Day
(MG/Day)
Size of
Population
Served
Primary
Water
Source
Treatment
Method
Min.
Max.
Estimated
Mean
Std Error
of Mean
Mean
Std Error
Mean
Std Error
10,001-
50,000
Ground
Any
526
28
2,776
748.25
139.55
40,866.72
7,547.49
2.12
0.39
Surface
Any
938
79
7,061
1,482.26
78.42
58,655.62
2,416.70
4.26
0.21
Subtotal
1,464
28
7,061
1,218.54
88.45
52,264.40
3,593.76
3.49
0.25
More than
50,000
Ground
Conventional
Filtration
14
[CBI Redacted]
Membrane
19
37
4,462
2,265.77
215.84
28,648.91
4,640.99
6.21
0.59
Other
2
[CBI Redacted]
Softening
66
840
17,000
4,243.80
1,064.31
48,447.97
11,779.86
11.68
2.93
Ion Exchange
8
[CBI Redacted]
None
2
[CBI Redacted]
Subtotal
a
111
37
17,000
4,170.62
642.52
42,127.70
6,421.41
12.05
1.92
Surface
Conventional
Filtration
383
60
55,000
8,668.41
414.86
55,553.16
1,209.13
24.62
1.23
Membrane
19
2,165
7,000
4,355.71
265.22
54,155.94
3,006.79
12.19
0.68
Other
4
[CBI Redacted]
Softening
168
3
44,000
10,003.66
665.05
61,044.84
3,625.78
27.63
1.84
None
2
[CBI Redacted]
Subtotal
a
576
3
55,000
9,128.09
353.52
57,329.19
1,381.09
25.66
1.02
Subtotal
a
688
3
55,000
8,326.38
364.13
54,870.84
1,774.75
23.46
1.04
Total
a
2,151
3
55,000
3,490.24
172.10
53,097.43
2,529.14
9.87
0.49
a – CBI redacted water production estimates are included in subtotal and total rows.
3-10

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-5. Operating Days per WTP in 2006 (National Estimates Based on Responses to
Question 2c)
Classification
Estimated
Number of
WTPs
Operating Days in 2006
Size of
Population
Served
Primary
Water
Source
Treatment
Method
Minimum
Reported
Maximum
Reported
Est. Mean
Std. Error
of Mean
10,001-
50,000
Ground
Any
526
92
365
347.08
5.79
Surface
Any
938
73
365
350.42
4.07
Subtotal
1,464
73
365
349.22
3.32
More than
50,000
Ground
Conventional
Filtration
14
[CBI Redacted]
Membrane
19
365
365
365
0
Other
2
[CBI Redacted]
Softening
66
349
365
364.5
0.3
Ion Exchange
8
[CBI Redacted]
None
2
[CBI Redacted]
Subtotal
a
111
202
365
359.18
2.97
Surface
Conventional
Filtration
383
12
365
354.54
1.86
Membrane
19
292
365
354.75
4.77
Other
4
[CBI Redacted]
Softening
168
250
365
360.8
1.24
None
2
[CBI Redacted]
Subtotal
a
576
12
365
356.49
1.3
Subtotal
a
688
12
365
356.93
1.19
Total
a
2,151
12
365
351.68
2.31
a – CBI redacted operating days are included in subtotal and total rows.
3-11

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-6. WTP Age (National Estimates Based on Responses to Question 2d)
Classification
WTP Built (Year)
Last upgrade (Year)
Size of
Population
Served
Primary
Water
Source
Treatment
Method
Earliest
Most
Recent
Median
Earliest
Most
Recent
Median
10,001-
50,000
Ground
Any
1928
2006
1991
1946
2006
1992
Surface
Any
1881
2006
1966
1912
2007
1994
Subtotal
1881
2006
1973
1912
2007
1994
More than
50,000
Ground
Conventional
Filtration
[CBI Redacted]
Membrane
1977
2002
1992
1986
2002
2002
Other
[CBI Redacted]
Softening
1953
2003
1992
1953
2006
2004
Ion Exchange
[CBI Redacted]
None
[CBI Redacted]
Subtotal
a
1928
2005
1992
1953
2006
2002
Surface
Conventional
Filtration
1873
2004
1967
1885
2007
1996
Membrane
1939
2006
2003
1998
2006
2003
Other
[CBI Redacted]
Softening
1867
2003
1956
1906
2006
1990
None
[CBI Redacted]
Subtotal
a
1867
2006
1965
1885
2007
1995
Subtotal
a
1867
2006
1967
1885
2007
1996
Total
a
1867
2006
1970
1885
2007
1994
a – CBI redacted WTP years built and upgraded are included in subtotal and total rows.
3.2.2.2 Source Water Treatment Operations (Question 2f)
This subsection summarizes the responses to Question 2f which collected data
about source water treatment operations employed at the WTP, chemicals used in the operations,
and the amounts of the chemicals used. Section 3.2.1 describes the assignment of WTPs to each
treatment method based upon information in question 2f. Tables 3-7 through 3-10 provide
national estimates about source water treatment operations. If the respondent did not check a
particular box, EPA assumed that the answer was ‘no’ (e.g., if the respondent did not check the
box for presedimentation, EPA assumed that this procedure was not conducted at the WTP).
Each table provides the estimated total number of WTPs in the classification and the smaller
subset that performed each different operation in 2006. For example, an estimated 38 of the 526
3-12

Drinking Water Industry Report Section 3 – Industry Profile
ground water plants that serve less than 50,000 people use presedimentation (Table 3-7). The
discussion below identifies the specific portions of the question related to each table.
Table 3-7 estimates the number of WTPs that use presedimentation as part of their
source water treatment operations. Of the estimated 2,151 WTPs in the target population,
approximately 141 WTPs (7 percent) use presedimentation.
Figure 3-4. Question 2f: Source Water Treatment and Chemical Addition:
Presedimentation
Table 3-8 estimates the number of WTPs using primary disinfection and the type
of disinfection. The estimates are based upon responses to two parts of Question 2f as shown in
Figure 3-5. Based upon the responses, 93 percent of the WTPs in the target population perform
primary disinfection (i.e., 2,002 of the 2,151 WTPs). No respondent selected hydrogen peroxide,
which appeared as one option in the survey. Thus, it does not appear in the table. Table 3-8 also
shows the estimated 230 WTPs, by classification, that perform dechlorination.
□ Presedimentation
Average Amount Per Day
(number, e.g., 20)
Units
□ Polymer coagulant
□ tons □ lbs
□ Other (specify):___________
□ tons □ lbs
3-13

Drinking Water Industry Report Section 3 – Industry Profile
Figure 3-5. Question 2f: Source Water Treatment and Chemical Addition: Primary
Disinfection and Dechlorination
Table 3-9 provides information about disinfection residuals in the filter backwash
and filter-to-waste. The estimates are based upon responses to two parts of Question 2f as shown
in Figure 3-6. For each of these two items, WTPs were asked to check whether they had free
chlorine, chloramination, other, or no backwash or filter-to-waste at the plant. EPA estimates that
most WTPs generate filter backwash and filter-to-waste (i.e., 1,906 and 1,809, respectively, of
the 2,151 WTPs).
Figure 3-6. Question 2f: Source Water Treatment and Chemical Addition: Disinfection
Residuals
□ Primary Disinfection (Please indicate type)
□ Free chlorine
□ Chloramination
□ Ozone
□ Ultraviolet light
□ Hydrogen peroxide (H
2
O
2
)
□ Other (specify):__________________
Note: Primary disinfection is intended to remove or inactivate harmful microorganisms at the
treatment plant, often conducted at the head of the plant or prior to filtration. This
disinfection treatment is different from secondary disinfection, which is conducted as one of
the final steps prior to distribution of the finished water. Secondary disinfection provides a
residual level of disinfection to help protect finished water as it travels through the system’s
distribution network.
□ Dechlorination
Average Amount Per
Day (number, e.g., 20)
Units
□ Sodium metabisulfite (Na
2
S
2
O
5
)
□ tons □ lbs
□ Other (specify):_______________
□ tons □ lbs
□ What type of disinfection residual is in the filter backwash? (Please indicate
type)
□ Free chlorine
□ Chloramination
□ Other (specify):__________________
□ No filter backwash at this plant
□ What type of disinfection residual is in the filter-to-waste? (Please indicate
type)
□ Free chlorine
□ Chloramination
□ Other (specify):__________________
□ No filter to-waste at this plant
3-14

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-10 provides the estimated number of WTPs using different types of
chemicals for primary disinfection. The questionnaire identified five primary categories that the
WTP could select, with one of the categories (ammonia) subdivided into four options as shown
in Figure 3-7. For the sake of summary, all ammonia responses were combined. EPA estimated
that approximately two-thirds of the WTPs (i.e., 1,418 of the 2,151 WTPs) use chlorine gas as a
primary disinfectant.
Figure 3-7. Question 2f: Source Water Treatment and Chemical Addition: Primary
Disinfectant
Please indicate below the type and
amount of the chemicals used for primary
disinfection.
Average Amount Per Day
(number, e.g., 20)
Units
□ Chlorine dioxide (ClO
2
)
□ tons □ lbs
□ Chlorine gas (Cl
2
, gas)
□ tons □ lbs
□ Calcium hypochlorite (Ca(OCl)
2
)
□ tons □ lbs
□ Sodium hypochlorite (NaOCl)
□ tons □ lbs
□ Ammonia (Please indicate form)
□ tons □ lbs
□ Anhydrous (NH
3
)
□ tons □ lbs
□ Ammonium sulfate ((NH
4
)
2
SO
4
)
□ tons □ lbs
□ Aqua ammonia (NH
4
+
)
□ tons □ lbs
□ Other (specify):_____________
□ tons □ lbs
□ Other (specify):_______________
□ tons □ lbs
3-15

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-7. Estimated Number of WTPs Using Presedimentation (National Estimates
Based on Responses to Question 2f)
Classification
Estimated Number of
WTPs in Classification
Estimated Number of
WTPs with
Presedimentation
Size of
Population
Served
Primary
Water
Source
Treatment Method
10,001-50,000
Ground
Any
526
38
Surface
Any
938
66
Subtotal
1,464
104
More than
50,000
Ground
Conventional Filtration
14
[CBI Redacted]
Membrane
19
[CBI Redacted]
Other
2
[CBI Redacted]
Softening
66
[CBI Redacted]
Ion Exchange
8
[CBI Redacted]
None
2
[CBI Redacted]
Subtotal
111
[CBI Redacted]
Surface
Conventional Filtration
383
[CBI Redacted]
Membrane
19
[CBI Redacted]
Other
4
[CBI Redacted]
Softening
168
[CBI Redacted]
None
2
[CBI Redacted]
Subtotal
576
[CBI Redacted]
Subtotal
a
688
37
Total
a
2,151
141
a – CBI redacted WTP estimates are included in subtotal and total rows.
3-16

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-8. Estimated Numbers of WTPs Using Various Primary Disinfection Methods (National Estimates Based on
Responses to Question 2f)
Classification
Estimated
Number of
WTPs in
Classification
Estimated
Number of
WTPs with
Primary
Disinfection
Estimated Number of WTPs Using:*
Estimated
Number of
WTPs that
Dechlorinate
Size of
Population
Served
Primary
Water
Source
Treatment
Method
Free
Chlorine
Chloramination
Ozone
UV
Other
10,001-
50,000
Ground
Any
526
452
331
118
0
0
0
64
Surface
Any
938
895
771
74
7
2
68
79
Subtotal
1,464
1,347
1,102
192
7
2
68
143
More than
50,000
Ground
Conventional
Filtration
14
[CBI Redacted]
[CBI Redacted]
Membrane
19
11
5
6
0
0
0
Other
2
[CBI Redacted]
Softening
66
66
21
44
0
0
0
Ion Exchange
8
[CBI Redacted]
None
2
[CBI Redacted]
Subtotal
111
101
43
51
2
2
4
Surface
Conventional
Filtration
383
377
316
37
46
2
19
Membrane
19
15
12
0
0
0
6
Other
4
[CBI Redacted]
Softening
168
159
122
39
10
0
10
None
2
[CBI Redacted]
Subtotal
576
554
454
76
56
2
35
Subtotal
a
688
655
496
126
58
4
39
87
Total
a
2,151
2,002
1,599
318
65
6
107
230
a – CBI redacted WTP estimates are included in subtotal and total rows.
3-17

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-9. Disinfection Residuals in Filter Backwash and Filter-to-Waste (National Estimates Based on Responses to
Question 2f)
Classification
Estimated
Number of
WTPs in
Classification
Estimated
Number of
WTPs with
Primary
Disinfection
Est. Number of WTPs Backwash
a
Est. Number of WTPs with Filter-to-Waste
a
Size of
Population
Served
Primary
Water
Source
Treatment
Method
Total
Free
chlorine
Chlora-
mination
Other
None
Total
Free
Chlorine
Chlora-
mination
Other
None
10,001-
50,000
Ground
Any
526
452
433
315
63
2
52
423
254
19
0
169
Surface
Any
938
895
847
674
114
52
15
775
464
56
69
201
Subtotal
1,464
1,347
1,279
989
178
54
67
1,198
718
75
69
370
More than
50,000
Ground
Conventional
Filtration
14
[CBI Redacted]
Membrane
19
11
5
0
0
0
5
5
0
0
0
5
Other
2
[CBI Redacted]
Softening
66
66
66
17
48
0
0
66
4
44
2
17
Ion Exchange
8
[CBI Redacted]
None
2
[CBI Redacted]
Subtotal
b
111
101
95
32
56
0
7
95
18
44
2
33
Surface
Conventional
Filtration
383
377
362
263
87
15
10
352
221
23
29
82
Membrane
19
15
15
13
2
0
0
15
6
0
0
9
Other
4
[CBI Redacted]
Softening
168
159
152
88
58
11
0
146
62
18
10
58
None
2
[CBI Redacted]
Subtotal
b
576
554
532
368
147
27
10
516
293
41
39
148
Subtotal
b
688
655
627
400
203
27
17
611
311
86
41
181
Total
b
2,151
2,002
1,906
1,388
380
80
84
1,809
1,028
161
110
552
a – WTPs may have more than one method of backwash or filter-to-waste. As a result, the sum of the number of WTPs in each of the three chemical categories may exceed the
value in the corresponding total column.
b – CBI redacted WTP estimates are included in subtotal and total rows.
3-18
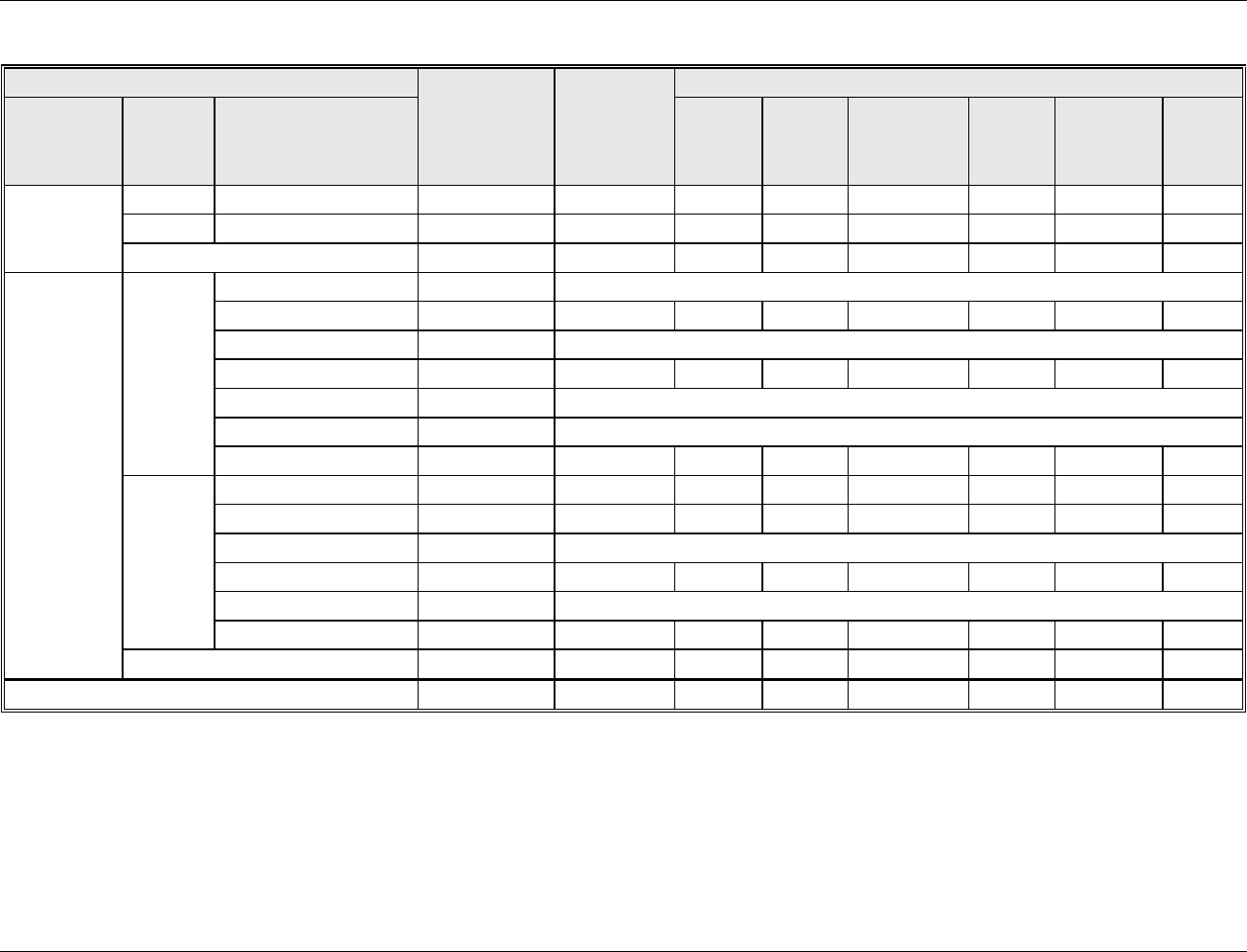
Drinking Water Industry Report Section 3 – Industry Profile
Table 3-10. Primary Disinfectants (National Estimates Based on Responses to Question 2f)
Classification
Estimated
Number of
WTPs in
Classification
Estimated
Number of
WTPs with
Primary
Disinfection
Estimated Number of WTPs Using Various Chemicals
a
Size of
Population
Served
Primary
Water
Source
Treatment Method
ClO
2
Cl
2
gas
Ca(OCl)
2
)
NaOCl
Ammonia
Other
10,001-
50,000
Ground
Any
526
452
0
330
.
102
44
0
Surface
Any
938
895
43
648
8
249
185
29
Subtotal
1,464
1,347
43
979
8
351
228
29
More than
50,000
Ground
Conventional Filtration
14
[CBI Redacted]
Membrane
19
11
0
0
0
5
0
0
Other
2
[CBI Redacted]
Softening
66
66
0
40
0
25
25
0
Ion Exchange
8
[CBI Redacted]
None
2
[CBI Redacted]
Subtotal
b
111
101
4
59
0
36
35
0
Surface
Conventional Filtration
383
377
31
239
2
116
129
23
Membrane
19
15
4
8
0
7
2
0
Other
4
[CBI Redacted]
Softening
168
159
4
131
0
24
67
14
None
2
[CBI Redacted]
Subtotal
b
576
554
39
380
2
147
198
37
Subtotal
b
688
655
42
439
2
182
233
37
Total
b
2,151
2,002
85
1,418
10
533
462
66
a – WTPs may use more than one chemical as primary disinfectant (e.g., ammonia and chlorine source to produce chloramines). As a result, the sum of the number of WTPs in
each of the chemical categories may exceed the value in the corresponding total column.
b – CBI redacted WTP estimates are included in subtotal and total rows.
3-19

Drinking Water Industry Report Section 3 – Industry Profile
3.2.2.3 Residuals Treatment and Pollution Prevention Practices (Questions 2h and
2i)
This subsection summarizes the responses to Questions 2h and 2i that address
residuals treatment and pollution prevention practices.
9
The responses are used to estimate the
number of WTPs within the target population with the different practices. If the respondent did
not check a particular box, EPA assumed that the answer was ‘no’ (e.g., if the respondent did not
check drying as a residuals treatment option, EPA assumed that drying operations were not
conducted at the plant).
Table 3-11 estimates different residuals management practices based upon the
responses to question 2h as shown in Figure 3-8. In the table, the term “non-mechanical
dewatering” also includes sedimentation tanks and ponds, thickening, evaporation ponds, and
drying. After excluding WTPs with pH adjustment, aeration, and hydrogen sulfide removal, EPA
estimates that approximately three-fourths of the WTPs in the target population treat residuals. In
other words, an estimated 522 of the 2,151 WTPs do not treat residuals.
Figure 3-8. Question 2h: Residuals Treatment
Table 3-12 estimates different pollution prevention practices based upon the
responses to question 2i as shown in Figure 3-9. The responses options include no pollution
prevention, recovery of treatment chemicals, recycling filter backwash, optimizing surface water
intake to reduce suspended solids intake, reuse of precipitative softening chemicals by recycling
9
Because few plants had affirmative responses, EPA did not provide national estimates for question 2g (“Is the
primary water treatment objective of the plant to remove salt from the source water (i.e., desalination)?”
2.h. Please indicate () below which residual treatment options were performed at the water
treatment plant in 2006. Treatment of residuals refers to any activity designed to change the
character or composition of liquid and solid residuals streams from water treatment processes as
needed to render it amenable to recycle/recovery, reduce its volume, or prepare it for
transportation, storage, disposal, or discharge.
□ No treatment □ Thickening □ Aeration
□ Drying □ Mechanical dewatering □ Hydrogen sulfide removal
□ pH adjustment □ Non-mechanical dewatering □ Evaporation ponds
□ Equalization of residuals prior to treatment or disposal □ Dechlorination
□ Sedimentation tanks and ponds
□ Other (specify):_______________________________
3-20

Drinking Water Industry Report Section 3 – Industry Profile
softening residuals to the head of the plant, recycling filter-to-waste, and other. EPA estimates
that approximately half of the WTPs in the target population (i.e., 1,036 of the 2,151 WTPs)
practice pollution prevention.
Figure 3-9. Question 2i: Pollution Prevention
2.i. Please indicate () below which pollution prevention practices were performed at the water
treatment plant in 2006. Pollution prevention refers to the use of materials, processes, or practices
that reduce or eliminate the creation of pollutants or residuals.
□ No pollution prevention
□ Recovery of treatment chemicals
□ Recycling filter backwash
□ Optimizing surface water intake to reduce suspended solids intake
□ Reuse of precipitative softening chemicals by recycling softening residuals to head of the plant
□ Recycling filter-to-waste
□ Other (specify):_______________________________
3-21

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-11. Residuals Treatment Methods (National Estimates Based on Responses to Question 2h)
Classification
Estimated
Number of
WTPs in
Classification
Estimated Number of WTPs With:
Size of
Population
Served
Primary
Water
Source
Treatment
Method
No
treatment
Equalization
only
Mechanical
dewatering
Non-mechanical
dewatering
Other
10,001-50,000
Ground
Any
526
253
31
8
225
14
Surface
Any
938
193
84
40
651
25
Subtotal
1,464
447
115
47
876
39
More than
50,000
Ground
Conventional
Filtration
14
[CBI Redacted]
Membrane
19
6
5
0
6
2
Other
2
[CBI Redacted]
Softening
66
2
0
15
61
0
Ion Exchange
8
[CBI Redacted]
None
2
[CBI Redacted]
Subtotal
a
111
12
7
19
89
2
Surface
Conventional
Filtration
383
32
25
88
308
8
Membrane
19
4
0
9
11
2
Other
4
[CBI Redacted]
Softening
168
27
12
28
123
6
None
2
[CBI Redacted]
Subtotal
a
576
63
37
129
448
16
Subtotal
a
688
75
44
148
537
18
Total
a
2,151
522
159
195
1,413
57
a – CBI redacted WTP estimates are included in subtotal and total rows.
3-22
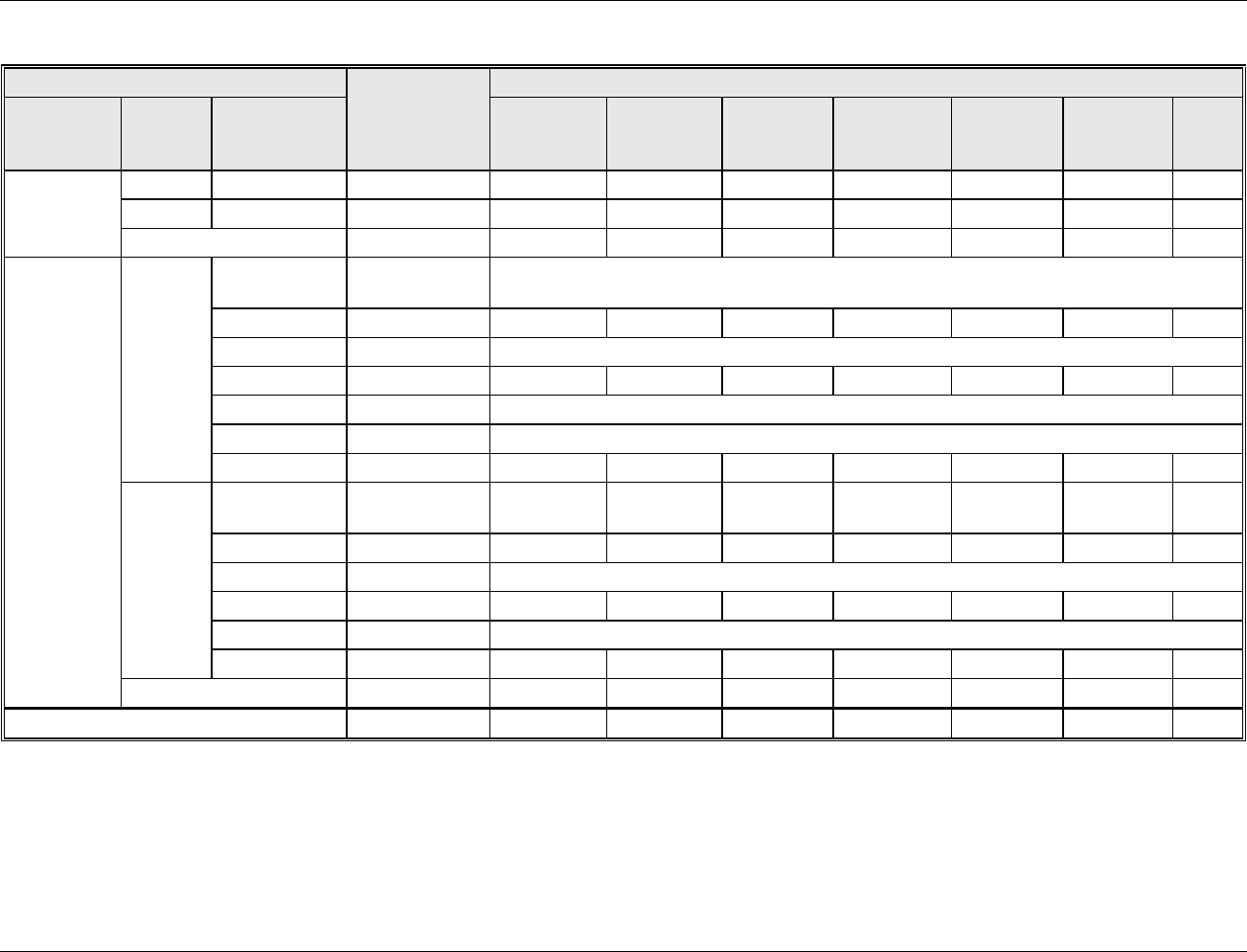
Drinking Water Industry Report Section 3 – Industry Profile
Table 3-12. Pollution Prevention Methods (National Estimates Based on Responses to Question 2i)
Classification
Estimated
Number of
WTPs in
Classification
Estimated Number of WTPs With:
Size of
Population
Served
Primary
Water
Source
Treatment
Method
No pollution
prevention
Recovery of
treatment
chemicals
Recycling
filter
backwash
Optimizing
surface
water intake
Recycle
softening
chemicals
Recycling
filter-to-
waste
Other
10,001-
50,000
Ground
Any
526
368
5
138
0
25
84
15
Surface
Any
938
514
39
285
140
31
159
42
Subtotal
1,464
882
43
423
140
56
243
57
More than
50,000
Ground
Conventional
Filtration
14
[CBI Redacted]
Membrane
19
11
0
6
2
6
0
0
Other
2
[CBI Redacted]
Softening
66
27
4
36
0
16
12
2
Ion Exchange
8
[CBI Redacted]
None
2
[CBI Redacted]
Subtotal
a
111
46
4
59
2
22
20
4
Surface
Conventional
Filtration
383
111
0
222
82
0
157
42
Membrane
19
10
0
7
2
0
2
5
Other
4
[CBI Redacted]
Softening
168
64
4
80
27
16
40
12
None
2
[CBI Redacted]
Subtotal
a
576
187
4
310
110
16
198
61
Subtotal
a
688
233
8
369
112
39
219
65
Total
a
2,151
1,115
51
792
253
95
461
122
a – CBI redacted WTP estimates are included in subtotal and total rows.
3-23

Drinking Water Industry Report Section 3 – Industry Profile
3.2.2.4 Residuals Discharge Practices (Question 2k)
This subsection summarizes the responses to Question 2k which collected data
about direct, indirect, and zero discharge streams. Tables 3-13 through 3-20 present national
estimates based upon the survey responses. The following paragraphs describe the tables and
identify the specific portions of the question related to each table. If the respondent did not check
a particular box, EPA assumed that the answer was ‘no’ (e.g., if a WTP did not check the box for
direct discharge, EPA assumed that none of its residuals were discharged in this manner).
Table 3-13 shows the estimated number of WTPs with each of the three discharge
methods: direct, indirect, and zero. It also estimates the number of WTPs that use one, two, or all
three discharge methods in their operations. The columns “Total Direct,” “Total Indirect,” and
“Total Zero” include WTPs that are estimated to discharge at least some of the residuals by that
method. The table also provides mutually exclusive estimates for each of the seven possible
combinations of discharge methods (e.g., “Direct Only” and “Direct and Zero”). For example, a
WTP that discharges some residuals to a stream and the rest to a POTW will form the basis of
the national estimates in the columns “Total Direct,” “Total Indirect,” and “Direct and Indirect.”
It will not be part of the estimates for any of the other columns for direct and/or indirect
dischargers. Figure 3-10 shows the portions of Question 2.k that were used to determine the
discharge type. EPA estimates that approximately 70 percent of the target population uses zero
discharge methods for some or all of its residuals (i.e., 1,502 of 2,151 WTPs). (See Table 3-20
for more details about zero discharge.)
3-24

Drinking Water Industry Report Section 3 – Industry Profile
Figure 3-10. Question 2k: Residuals Discharge Method
Tables 3-14a and 3-14b provide national estimates of the types of residuals
discharged in 2006. Although they are located under different sections of question 2k, the
choices are essentially the same for each discharge method and are shown in Figure 3-11. EPA
estimated the number of WTPs with residuals from four management practices: water treatment
operations, presedimentation operations, dewatering operations, and brines. EPA divided the
information into two tables to be more readable. The tables do not include national estimates for
the other survey options: residuals from stormwater, ion exchange resins, and other management
practices.
Dewatering operations generate more residuals than other types (1,402 of the
2,151 WTPs have residuals from dewatering operations). Some facilities indicated that they
operate presedimentation in Questions 2f (treatment operations) but not in Question 2k (residuals
discharge), which resulted in 141 WTPs indicating presedimentation operations, but only 70
WTPs with residuals from presedimentation. EPA chose to use the responses in Question 2f to
represent the number of WTPs operating presedimentation (141 WTPs) for the following
reasons:
• For Question 2f, plants would indicate operating presedimentation. The
residuals from presedimentation might then be discharged (directly or
indirectly), managed via zero discharge method (e.g., evaporation lagoon),
or sent to the residuals treatment plant for dewatering.
2.k. Please indicate () in 2.k.i, 2.k.ii, and 2.k.iii below the method(s) of residuals discharge
performed in 2006 at the water treatment plant and identify the year that this discharge method
started. Please select all categories that apply. (See Definitions of Key Terms on page 26 for
explanations of discharge types, pollutants, and residuals.)
i. Direct discharge of treated and/or untreated residuals. Do not select direct
discharge if your plant only discharges non-contact stormwater to surface waters.
Select direct discharge if your plant has a permit that regulates or monitors the
discharge of treated and/or untreated residuals to surface waters.
ii. Indirect discharge of treated and/or untreated residuals. Select indirect discharge
if your plant has a permit that regulates or monitors the discharge of treated and/or
untreated residuals to a treatment works (POTW, PrOTW, FOTW). Indirect
discharge does not include spent filter backwash discharged to surface water.
iii. Zero discharge.
3-25

Drinking Water Industry Report Section 3 – Industry Profile
• Plants may not indicate presedimentation residuals in Question 2k. If the
WTP dewaters the residuals from presedimentation, the WTP could select
“discharges from residuals treatment” instead of presedimentation
residuals. The WTP would make this selection especially if the residuals
from presedimentation are commingled with other waste streams.
EPA used responses to Question 2k to represent the discharges from
presedimentation that are directly discharged, indirectly discharged, or managed via zero
discharge method.
Figure 3-11. Question 2k: Type of Residuals Discharged
Table 3-15 presents the number of direct and indirect WTPs with different
discharge frequencies: continuous, batch, and emergency. Because a WTP could discharge more
than one of these types of releases, the sum of the estimated number of WTPs within each of the
three categories may be greater than the total number of direct or indirect discharging WTPs. As
shown in Figure 3-12, question 2k uses slightly different ways to collect the information from
direct and indirect dischargers, but the three discharge frequencies (continuous, batch,
emergency) were the same. As shown in the tables, batch discharges are estimated to be the most
common practice for both direct and indirect dischargers.
Types of Residuals Disposed of by the Specified Residuals Management Option(s) in 2006.
Please check all that apply.
Residuals from water treatment operations including coagulation, filter backwashing
operations, filter-to-waste, precipitative softening, iron and manganese removal, and slow sand and
diatomaceous earth filtration. These include accumulated residuals for batch discharge.
Residuals from presedimentation water treatment operations.
Discharges from residuals treatment including mechanical dewatering (e.g., thickener decant,
centrate, and filtrate from belt or plate-and-frame presses) and non-mechanical dewatering (e.g.,
discharges from dewatering lagoons).
Concentrate (brines) from ion exchange regeneration and salt water conversion, membrane
reject water and spent backwash, activated alumina waste regenerate, and membrane cleaning fluid.
Stormwater collected from areas associated with water treatment operations.
Stormwater collected from areas not associated with water treatment operations.
Ion exchange resins, spent GAC, and spent filter media.
Other
3-26

Drinking Water Industry Report Section 3 – Industry Profile
Figure 3-12. Question 2k: Frequency of Residuals Discharge
For direct dischargers, Tables 3-16 and 3-17 provide more information about
discharge practices. Table 3-16 presents the estimated number of batch and emergency
discharges in 2006 by direct dischargers. Table 3-17 presents the number of WTPs discharging
into different types of waterbodies: river, creek, wetland, ocean, lake and other. Based upon the
responses, the most common destinations for direct dischargers are likely to be rivers or creeks.
Figure 3-13 shows the portions of question 2k used to derive the national estimates in Tables 3-
16 and 3-17.
Figure 3-13. Question 2k: Direct Discharge—Continuous, Batch or Emergency and Type of
Receiving Stream
If the water treatment plant directly discharged its residuals to surface water bodies in 2006, please
indicate () below the frequency of the discharge. In the blank spaces below the batch and
emergency discharge categories, please specify the number of times residuals were discharged to
surface waters in 2006. Please indicate () below both ‘Continuous discharge’ and ‘Batch
(intermittent) discharge’ if you are doing both types of discharges (e.g., continuous filter backwash
and batch discharge of residuals in settling basins).
Continuous discharge
Batch (intermittent) discharge
Residuals were discharged ________ times in 2006.
Emergency discharge only
Residuals were discharged ________ times in 2006.
If the water treatment plant indirectly discharged its residuals to a treatment works (POTW,
PrOTW, FOTW) in 2006, please indicate () below the frequency and volume of the discharge to
the nearest 1,000 gallons. In the blank spaces below the batch and emergency discharge categories,
please specify the number of times residuals were discharged in 2006.
Continuous discharge
Volume of discharge ________ gallons per day.
Batch (intermittent) discharge
Residuals were discharged ________ times in 2006.
Volume of discharge ________ gallons per day.
Emergency discharge only
Residuals were discharged ________ times in 2006.
Volume of discharge ________ gallons per day
If the water treatment plant directly discharged its residuals to surface water bodies in 2006, …
Continuous …
Batch …
Emergency discharge only
Residuals were discharged ________ times in 2006.
Type of Receiving Water (See Definitions of Key Terms on page 26 for explanations of types.)
River Creek Wetland Ocean Lake
Other (specify):____________________
3-27

Drinking Water Industry Report Section 3 – Industry Profile
For indirect dischargers, Tables 3-18 and 3-19 provide more information about
discharge practices. They provide national estimates for the number of WTPs and daily volumes
for continuous and batch discharges. (Only one WTP provided volumes for emergency
discharges.) The information was collected from the portion of question 2k shown in Figure 3-
14.
Figure 3-14. Question 2k: Indirect Discharge—Continuous, Batch or Emergency and
Volume Discharged
Table 3-20 provides the national estimates for the number of WTPs using
alternative discharge methods by one or more of eight “zero discharge” disposal methods:
recycling, evaporation, composting, landfill disposal, spray irrigation, underground injection,
land application, and other. The relevant part of the question is shown in Figure 3-15. Based
upon the responses, more than half of the WTPs recycle and/or use landfills to reduce or
eliminate wastewater discharges. Of the estimated 1,502 WTPs using zero discharge methods, an
estimated 790 recycle waste streams and 792 use landfills.
If the water treatment plant indirectly discharged its residuals to a treatment works (POTW,
PrOTW, FOTW) in 2006, please indicate () below the frequency and volume of the discharge to
the nearest 1,000 gallons. In the blank spaces below the batch and emergency discharge categories,
please specify the number of times residuals were discharged in 2006.
Continuous discharge
Volume of discharge ________ gallons per day.
Batch (intermittent) discharge
Residuals were discharged ________ times in 2006.
Volume of discharge ________ gallons per day.
Emergency discharge only
Residuals were discharged ________ times in 2006.
Volume of discharge ________ gallons per day
3-28

Drinking Water Industry Report Section 3 – Industry Profile
Figure 3-15. Question 2k: Zero Discharge Methods
If the water treatment plant operated as a zero-discharge plant in 2006, please identify () the
disposal method(s) for the residuals.
Recycle (i.e., return to water treatment plant pre-coagulation)
Evaporation
Composting
Landfill disposal
Spray irrigation
Underground injection
Land application (e.g., soil amendment)
Other (specify):______________________________
Other (specify): ______________________________
Other (specify): ______________________________
3-29

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-13. Estimated Numbers of WTPs Using Direct, Indirect, or Zero Residuals Discharge Practices (National Estimates
Based on Responses to Question 2k)
Classification
Estimated
Number of
WTPs in
Classification
Estimated Number
Size of
Population
Served
Primary
Water
Source
Treatment
Method
Direct
only
Indirect
only
Zero
only
Direct
and
Indirect
Direct
and
Zero
Indirect
and Zero
Direct,
Indirect,
and Zero
Total
Direct
Total
Indirect
Total
Zero
a
10,001-
50,000
Ground
Any
526
49
195
107
8
63
103
0
121
307
273
Surface
Any
938
49
173
206
50
279
154
28
406
405
666
Subtotal
1,464
98
368
312
59
342
257
28
527
711
939
More than
50,000
Ground
Conventional
Filtration
14
[CBI Redacted]
Membrane
19
0
4
13
2
0
0
0
2
6
13
Other
2
[CBI Redacted]
Softening
66
2
0
32
0
29
2
0
31
2
64
Ion Exchange
8
[CBI Redacted]
None
2
[CBI Redacted]
Subtotal
b
111
4
5
54
2
34
13
0
40
20
100
Surface
Conventional
Filtration
383
15
18
123
32
103
74
18
167
142
318
Membrane
19
0
6
2
0
4
7
0
4
12
13
Other
4
[CBI Redacted]
Softening
168
14
17
40
8
59
21
8
90
55
129
None
2
[CBI Redacted]
Subtotal
b
576
32
41
165
40
168
104
26
266
211
463
Subtotal
b
688
36
47
219
42
202
117
26
305
231
563
Total
b
2,151
134
415
531
100
544
374
54
832
943
1,502
a – Number of WTPs using one or more zero discharge method (e.g., landfill disposal, recycling). WTP may also discharge some residuals directly or indirectly.
b – CBI redacted WTP estimates are included in subtotal and total rows.
3-30

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-14a. Estimated Numbers of WTPs by Types of Residuals Discharged and Discharge Practice (National Estimates
Based on Responses to Question 2k)
Classification
Estimated
Number of
WTPs in
Classification
Estimated Number of WTPs with Residuals from:
Source water treatment operations
Presedimentation operations
Size of
Population
Served
Primary
Water
Source
Treatment
Method
Total
Direct
Indirect
Zero
a
Total
Direct
Indirect
Zero
a
10,001-50,000
Ground
Any
526
281
45
179
84
0
0
0
0
Surface
Any
938
351
91
227
61
39
15
15
8
Subtotal
1,464
632
136
407
145
39
15
15
8
More than
50,000
Ground
Conventional
Filtration
14
[CBI Redacted]
Membrane
19
0
0
0
0
0
0
0
0
Other
2
[CBI Redacted]
Softening
66
26
2
0
24
0
0
0
0
Ion Exchange
8
[CBI Redacted]
None
2
[CBI Redacted]
Subtotal
b
111
34
4
2
29
0
0
0
0
Surface
Conventional
Filtration
383
150
35
69
81
13
0
0
13
Membrane
19
6
0
6
0
0
0
0
0
Other
4
[CBI Redacted]
Softening
168
86
35
37
28
17
8
2
6
None
2
[CBI Redacted]
Subtotal
b
576
244
72
111
110
32
8
2
21
Subtotal
b
688
278
76
114
138
32
8
2
21
Total
b
2,151
910
212
520
283
70
24
18
29
a – Number of WTPs using one or more zero discharge method (e.g., landfill disposal, recycling). WTP may also discharge some residuals directly or indirectly.
b – CBI redacted WTP estimates are included in subtotal and total rows.
3-31

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-14b. Estimated Numbers of WTPs by Types of Residuals Discharged and Discharge Practice (National Estimates
Based on Responses to Question 2k)
Classification
Estimated Number
of WTPs in
Classification
Estimated Number of WTPs with Residuals from:
Dewatering Operations
Concentrates (i.e., Brines)
Size of
Population
Served
Primary
Water
Source
Treatment
Method
Total
Direct
Indirect
Zero
a
Total
Direct
Indirect
Zero
a
10,001-50,000
Ground
Any
526
197
44
69
173
107
32
78
25
Surface
Any
938
662
289
161
585
21
2
17
9
Subtotal
1,464
859
334
230
758
127
34
95
34
More than
50,000
Ground
Conventional
Filtration
14
[CBI Redacted]
Membrane
19
6
0
0
6
19
2
6
13
Other
2
[CBI Redacted]
Softening
66
60
29
2
60
0
0
0
0
Ion Exchange
8
[CBI Redacted]
None
2
[CBI Redacted]
Subtotal
b
111
86
32
4
85
30
4
14
13
Surface
Conventional
Filtration
383
318
129
95
266
0
0
0
0
Membrane
19
13
4
2
13
7
0
5
7
Other
4
[CBI Redacted]
Softening
168
122
53
25
114
0
0
0
0
None
2
[CBI Redacted]
Subtotal
b
576
457
186
124
396
7
0
5
7
Subtotal
b
688
542
218
128
481
36
4
19
20
Total
b
2,151
1,402
552
358
1,239
164
38
113
54
a – Number of WTPs using one or more zero discharge method (e.g., landfill disposal, recycling). WTP may also discharge some residuals directly or indirectly.
b – CBI redacted WTP estimates are included in subtotal and total rows.
3-32

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-15. Estimated Number of WTPs by Discharge Frequency for Direct and Indirect Discharges (National Estimates
Based on Responses to Question 2k)
Classification
Estimated
Number of
WTPs in
Classification
Estimated
Number of
Direct and
Indirect
WTPs
Estimated Number of WTPs with:
Direct Discharge
a
Indirect Discharge
a
Size of
Population
Served
Primary
Water
Source
Treatment
Method
Total
Cont.
Batch
Emer-
gency
Total
Cont.
Batch
Emer-
gency
10,001-
50,000
Ground
Any
526
419
121
35
86
0
307
44
265
0
Surface
Any
938
732
406
158
220
35
405
129
271
4
Subtotal
1,464
1,151
527
194
306
35
711
173
537
4
More than
50
,000
Ground
Conventional
Filtration
14
8
[CBI Redacted]
Membrane
19
6
2
2
0
0
6
6
0
0
Other
2
2
[CBI Redacted]
Softening
66
33
31
2
27
2
2
0
2
0
Ion Exchange
8
8
[CBI Redacted]
None
2
0
[CBI Redacted]
Subtotal
b
111
57
40
8
29
2
20
7
13
0
Surface
Conventional
Filtration
383
260
167
78
76
15
142
48
102
0
Membrane
19
17
4
4
0
0
12
6
7
0
Other
4
4
[CBI Redacted]
Softening
168
129
90
44
45
8
55
24
31
0
None
2
2
[CBI Redacted]
Subtotal
b
576
412
266
126
123
25
211
78
141
0
Subtotal
b
688
469
305
135
152
27
231
85
154
0
Total
b
2,151
1,620
832
328
458
62
943
258
691
4
a – WTPs may use more than discharge flow type (continuous, batch or emergency). As a result, the sum of the number of WTPs in each of the three discharge
flow types may exceed the value in the corresponding total column.
b – CBI redacted WTP estimates are included in subtotal and total rows.
3-33

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-16. Estimated Number of Batch and Emergency Dischargers by Direct-Discharging WTPs (National Estimates Based
on Responses to Question 2k)
Classification
Estimated
Number of
WTPs in
Classification
Estimated
Number of
Direct
Dischargers
Estimated Number of WTPs and Frequency of Residual Discharges
Batch Discharge (Times in 2006)
Emergency Discharge (Times in 2006)
Size of
Population
Served
Primary
Water
Source
Treatment
Method
Estimated
Number of
WTPs
Min
Max
Median
Estimated
Number of
WTPs
Min
Max
Median
10,001-
50,000
Ground
Any
526
121
86
50
365
365
0
0
0
0
Surface
Any
938
406
220
2
19,000
365
35
0
218
2
Subtotal
1,464
527
306
2
19,000
365
35
0
218
2
More than
50,000
Ground
Conventional
Filtration
14
4
[CBI Redacted]
[CBI Redacted]
Membrane
19
2
0
-
-
-
0
-
-
-
Other
2
2
[CBI Redacted]
[CBI Redacted]
Softening
66
31
27
21
1,500
1,000
2
0
0
0
Ion Exchange
8
0
--
--
None
2
0
--
--
Subtotal
a
111
40
29
21
1,500
1,000
2
0
0
0
Surface
Conventional
Filtration
383
167
76
2
70,810
455
15
0
6
0
Membrane
19
4
0
-
-
-
0
-
-
-
Other
4
2
[CBI Redacted]
[CBI Redacted]
Softening
168
90
45
1
36,000
1,095
8
0
12
0
None
2
2
[CBI Redacted]
[CBI Redacted]
Subtotal
a
576
266
123
1
70,810
589
25
0
12
0
Subtotal
a
688
305
152
1
70,810
848
27
0
12
0
Total
a
2,151
832
458
1
70,810
365
62
0
218
1
a – CBI redacted WTP estimates are included in subtotal and total rows.
3-34

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-17. Estimated Numbers of WTPs Directly Discharging to Various Types of Receiving Waters (National Estimates
Based on Responses to Question 2k)
Classification
Estimated
Number of
WTPs in
Classification
Estimated
Number of
Direct
Dischargers
Estimated Number of WTPs Directly Discharging to Receiving
Waters
Size of
Population
Served
Primary
Water
Source
Treatment Method
River
Creek
Wetland
Ocean
Lake
Other
10,001-
50,000
Ground
Any
526
121
57
55
0
2
0
6
Surface
Any
938
406
167
157
0
8
67
0
Subtotal
1,464
527
224
212
0
10
67
6
More than
50,000
Ground
Conventional
Filtration
14
4
[CBI Redacted]
Membrane
19
2
0
0
0
0
2
0
Other
2
2
[CBI Redacted]
Softening
66
31
23
8
0
0
0
0
Ion Exchange
8
0
--
None
2
0
--
Subtotal
a
111
40
28
8
0
0
2
2
Surface
Conventional
Filtration
383
167
61
60
0
0
39
8
Membrane
19
4
0
2
0
0
0
0
Other
4
2
[CBI Redacted]
Softening
168
90
47
25
0
0
12
2
None
2
2
[CBI Redacted]
Subtotal
a
576
266
109
87
0
0
51
13
Subtotal
a
688
305
137
95
0
0
53
15
Total
a
2,151
832
361
307
0
10
119
21
a – CBI redacted WTP estimates are included in subtotal and total rows.
3-35

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-18. Estimated Number of WTPs with Indirect Discharge and
Release Volumes for Continuous Discharges (National Estimates Based on Responses to Question 2k)
Classification
Estimated
Number of
WTPs in
Classification
Estimated
Number of
Indirect
Dischargers
Continuous
Size of
Population
Served
Primary
Water
Source
Treatment Method
Est #
WTPs
Gallons/Day
Min.
Max.
Median
10,001-50,000
Ground
Any
526
307
44
720
1,000,000
50,000
Surface
Any
938
405
129
5000
610,000
80,000
Subtotal
1,464
711
173
720
1,000,000
1,000,000
More than
50,000
Ground
Conventional Filtration
14
4
[CBI Redacted]
Membrane
19
6
6
122,200
997,000
260,000
Other
2
0
0
-
-
-
Softening
66
2
0
-
-
-
Ion Exchange
8
8
[CBI Redacted]
None
2
0
0
-
-
-
Subtotal
a
111
20
7
122,020
997,000
260,000
Surface
Conventional Filtration
383
142
48
6,375
1,404,000
173,337
Membrane
19
12
6
111,233
341,000
226,117
Other
4
2
[CBI Redacted]
Softening
168
55
24
3,562
1,056,960
300,000
None
2
0
0
-
-
-
Subtotal
a
576
211
78
3,562
1,404,000
200,000
Subtotal
a
688
231
85
3,562
1,000,000
1,404,000
Total
a
2,151
943
258
720
1,000,000
99,800
a – CBI redacted WTP estimates are included in subtotal and total rows.
3-36

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-19. Estimated Number of WTPs with Indirect Discharge and Release Volumes for Batch Discharges (National
Estimates Based on Responses to Question 2k)
Classification
Estimated
Number of WTPs
in Classification
Estimated
Number of
Indirect
Dischargers
Batch
Size of
Population
Served
Primary
Water
Source
Treatment Method
Est # WTPs
Gallons/Day
Min.
Max.
Median
10,001-
50,000
Ground
Any
526
307
265
157
700,000
15,000
Surface
Any
938
405
271
110
1,234,000
60,000
Subtotal
1,464
711
537
110
1,234,000
16,000
More than
50,000
Ground
Conventional Filtration
14
4
[CBI Redacted]
Membrane
19
6
0
-
-
-
Other
2
0
0
-
-
-
Softening
66
2
2
270,000
270,000
270,000
Ion Exchange
8
8
[CBI Redacted]
None
2
0
0
-
-
-
Subtotal
a
111
20
13
1,600
270,000
51,305
Surface
Conventional Filtration
383
142
102
246
730,000
45,000
Membrane
19
12
7
8,000
2,265,900
8,000
Other
4
2
[CBI Redacted]
Softening
168
55
31
26,000
1,000,000
350,000
None
2
0
0
-
-
-
Subtotal
a
576
211
141
246
2,265,900
70,000
Subtotal
a
688
231
154
246
2,265,900
67,641
Total
a
2,151
943
691
110
2,265,900
25,000
a – CBI redacted WTP estimates are included in subtotal and total rows.
3-37

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-20. Estimated Number of WTPs Employing Various Zero Discharge Disposal Methods (National Estimates Based on
Responses to Question 2k)
Classification
Estimated
Number of
WTPs in
Classification
Estimated
Number of
WTPs using
Zero
Discharge
Methods
a
Estimated Number of WTPs Using Zero Discharge Methods
a
:
Size of
Population
Served
Primary
Water
Source
Treatment
Method
Recycle
Evaporation
Compost
Landfill
Spray
Irrigation
Underground
Injection
Land
Application
Other
10,001-
50,000
Ground
Any
526
273
151
74
0
143
0
3
52
48
Surface
Any
938
666
284
141
12
388
14
0
166
25
Subtotal
1,464
939
434
216
12
531
14
3
218
73
More than
50,000
Ground
Conventional
Filtration
14
11
[CBI Redacted]
Membrane
19
13
6
0
0
6
0
11
2
0
Other
2
2
[CBI Redacted]
Softening
66
64
36
7
0
16
0
0
48
4
Ion Exchange
8
8
[CBI Redacted]
None
2
2
[CBI Redacted]
Subtotal
b
111
100
59
12
0
37
0
11
50
13
Surface
Conventional
Filtration
383
318
211
69
10
165
8
0
74
17
Membrane
19
13
7
0
0
9
0
0
6
2
Other
4
2
[CBI Redacted]
Softening
168
129
77
20
8
46
0
0
48
15
None
2
2
[CBI Redacted]
Subtotal
b
576
463
297
88
18
224
8
0
130
33
Subtotal
b
688
563
356
101
18
261
8
11
180
46
Total
a,b
2,151
1,502
790
316
29
792
21
14
399
119
a – Number of WTPs using one or more zero discharge method (e.g., landfill disposal, recycling). WTP may also discharge some residuals directly or indirectly.
b – CBI redacted WTP estimates are included in subtotal and total rows.
3-38

Drinking Water Industry Report Section 3 – Industry Profile
3.2.2.5 Copper Usage (Question 3)
This subsection summarizes the responses to question 3 about the WTP’s usage of
copper-based chemicals to treat source water. For example, WTPs might use copper-based
chemicals to control nuisance algae in reservoirs. Tables 3-21 through 3-24 estimate copper
usage by the target population based upon the responses to the question shown in Figure 3-16. If
the respondent did not check a particular box, EPA assumed that the answer was ‘no.’
Tables 3-21 and 3-22 estimate the application rate of the copper sulfate and
chelated copper complexes for WTPs in the target population that use copper. The application
rate is expressed in pounds per acre-foot and was calculated as:
volumeReservoir
amount
Annual
Rate =
(Eq. 3-1)
Tables 3-23 and 3-24 estimate the metallic copper content of the treatments based
upon the responses from WTPs using copper. For each response, EPA calculated the amount of
metallic copper in one of two ways, depending upon whether the metallic content of the copper
was expressed by weight or by volume. For weight-based metallic copper, the metallic copper
was calculated as follows:
100
P
WW
W
cm
⋅=
(Eq. 3-2)
where W
m
is the weight of metallic copper (lbs), W
c
is the total weight of chemical (lbs), and P
W
is the percentage of metallic copper by weight. For volume-based metallic copper, the weight of
metallic copper was calculated as follows:
( ) ( )
/100P18.92/100P
8.92/100P
WW
VV
V
cm
−+⋅
⋅
⋅=
(Eq. 3-3)
where P
V
is the percentage of metallic copper by volume.
3-39

Drinking Water Industry Report Section 3 – Industry Profile
Figure 3-16. Question 3: Use of Copper-Based Chemicals to Treat Source Water
3. Were copper-based chemicals used at the plant to treat the source water in
2006?
Yes
No (Skip to Question 4.)
Please indicate () the type(s) of chemical(s) used at the plant to promote a
better source of drinking water (e.g., control nuisance algae).
Copper sulfate (CuSO4)
Chelated copper complexes (i.e., copper citrate, copper ethanolamine,
copper ethylene)
Other (specify):
Other (specify):
Other (specify):
If more than one chemical was selected above, please photocopy this page and
provide the following information for each chemical.
Name of chemical or product____________________________
Amount of this chemical used at this plant in 2006: ___________lbs
Volume of treatment reservoir: _____________acre-feet
Percent of metallic copper (label will note as Cu++ or Cu+2): _____________%
by weight
3-40

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-21. Estimated Number of WTPs Using Copper Sulfate and Application Rate (National Estimates Based on Responses
to Question 3)
Classification
Estimated
Number of
WTPs in
Classification
Estimated Number
of WTPs Using
Copper-based
Chemicals
Copper Sulfate
Size of
Population
Served
Primary
Water
Source
Treatment
Method
Estimated
Number of
WTPs
Application Rate (lbs/acre-ft)
Min.
Max.
Est. Mean
Std Err of
Mean
10,001-
50,000
Ground
Any
526
0
0
-
-
-
-
Surface
Any
938
95
87
0.02
1,000
102.89
88.61
Subtotal
1,464
95
87
0.02
1,000
102.89
88.64
More than
50,000
Ground
Conventional
Filtration
14
[CBI Redacted] [CBI Redacted]
Membrane
19
Other
2
Softening
66
Ion Exchange
8
None
2
Subtotal
a
111
5
5
0.46
4.11
2.18
1.25
Surface
Conventional
Filtration
383
[CBI Redacted] [CBI Redacted]
Membrane
19
Other
4
Softening
168
None
2
Subtotal
a
576
92
64
0
50,000
2,355.9
2,195.4
Subtotal
a
688
96
69
0
50,000
2,167.9
2,021.4
Total
a
2,151
191
156
0
50,000
978.12
8,81.43
a – CBI redacted WTP estimates are included in subtotal and total rows.
3-41

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-22. Estimated Number of WTPs Using Chelated Copper Complexes and Application Rate (National Estimates Based
on Responses to Question 3)
Classification
Estimated
Number of
WTPs in
Classification
Estimated
Number of
WTPs Using
Copper-based
Chemicals
Chelated copper complexes
Size of
Population
Served
Primary
Water
Source
Treatment
Method
Estimated Number
of WTPs
Application Rate (lbs/acre-ft)
Min
Max
Est. Mean
Std Err of
Mean
10,001-
50,000
Ground
Any
526
0
0
-
-
-
-
Surface
Any
938
95
18
0
0.03
0.01
0.01
Subtotal
1,464
95
18
0
0.03
0.01
0.01
More than
50,000
Ground
Conventional
Filtration
14
[CBI Redacted] [CBI Redacted]
Membrane
19
Other
2
Softening
66
Ion Exchange
8
None
2
Subtotal
a
111
5
2
0
0
0
0
Surface
Conventional
Filtration
383
[CBI Redacted] [CBI Redacted]
Membrane
19
Other
4
Softening
168
None
2
Subtotal
a
576
92
29
0
25.25
2.6
1.93
Subtotal
a
688
96
32
0
25.25
2.37
1.77
Total
a
2,151
191
50
0
25.25
1.45
1.19
a – CBI redacted WTP estimates are included in subtotal and total rows.
3-42

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-23. Estimated Number of WTPs Using Copper Sulfate and Amount of Metallic Copper Used in Pounds (National
Estimates Based on Responses to Question 3)
Classification
Estimated
Number of
WTPs in
Classification
Estimated
Number of
WTPs Using
Copper-
based
Chemicals
Copper Sulfate
Size of
Population
Served
Primary
Water
Source
Treatment
Method
Estimated
Number of
WTPs
Amount of metallic copper (lbs/yr)
Minimum
Maximum
Estimated
Mean
Std err of
mean
10,001-
50,000
Ground
Any
526
0
0
-
-
-
-
Surface
Any
938
95
87
1
2,000
515
164
Subtotal
1,464
95
87
1
2,000
515
164
More than
50,000
Ground
Conventional
Filtration
14
[CBI
Redacted]
[CBI Redacted]
Membrane
19
Other
2
Softening
66
Ion Exchange
8
None
2
Subtotal
a
111
5
5
1,969
3,465
2,674
512
Surface
Conventional
Filtration
383
[CBI
Redacted]
[CBI Redacted]
Membrane
19
Other
4
Softening
168
None
2
Subtotal
a
576
92
64
21
34,520
3,322
1,558
Subtotal
a
688
96
69
21
34,520
3,269
1,431
Total
a
2,151
191
156
1
34,520
1,666
655
a – CBI redacted WTP estimates are included in subtotal and total rows.
3-43

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-24. Estimated Number of WTPs Using Chelated Copper Complexes and
Amount of Metallic Copper Used in Pounds (National Estimates Based on Responses to Question 3)
Classification
Estimated
Number of
WTPs in
Classification
Estimated
Number of
WTPs Using
Copper-based
Chemicals
Chelated copper complexes
Size of
Population
Served
Primary
Water
Source
Treatment
Method
Estimated
Number of
WTPs
Amount of metallic copper (lbs/yr)
Minimum
Maximum
Estimated.
Mean
Std Err of
Mean
10,001-
50,000
Ground
Any
526
0
0
-
-
-
-
Surface
Any
938
95
18
0
24
12
8
Subtotal
1,464
95
18
0
24
12
8
More than
50,000
Ground
Conventional
Filtration
14
[CBI Redacted] [CBI Redacted]
Membrane
19
Other
2
Softening
66
Ion Exchange
8
None
2
Subtotal
a
111
5
2
0
0
0
0
Surface
Conventional
Filtration
383
[CBI Redacted] [CBI Redacted]
Membrane
19
Other
4
Softening
168
None
2
Subtotal
a
576
92
29
0
5,533
1,298
580
Subtotal
a
688
96
32
0
5,533
1,165
534
Total
a
2,151
191
50
0
5,533
717
398
a – CBI redacted WTP estimates are included in subtotal and total rows.
3-44

Drinking Water Industry Report Section 3 – Industry Profile
3.3 DRINKING WATER INDUSTRY ECONOMIC OVERVIEW
This economic overview compiles and analyzes economic and operational data
for public water systems (PWSs) and provides a general overview of the types and characteristics
of public drinking water systems. The purpose of this section is to provide an overview of the
financial characteristics of PWSs that operate WTPs serving at least 10,000 people, as well as the
variability of financial strength across drinking water systems. The remainder of this section is
organized as follows:
• Section 3.3.1 describes the major data sources used for this profile.
• Section 3.3.2 presents a general overview of PWSs, including population
served, ownership type, water source, and discharge characteristics.
• Section 3.3.3 reviews financial characteristics of PWSs.
• Section 3.3.4 provides an overview of water system customers, with focus
on water consumption and rate payments by residential customers.
3.3.1 Major Sources of Information
EPA used three primary sources of data to characterize the universe of PWSs: the
Safe Drinking Water Information System (SDWIS), the Community Water System Survey
(CWSS), and the responses to the EPA DWT Industry Questionnaire.
3.3.1.1 Safe Drinking Water Information System
As discussed in Section 2.3.1, the SDWIS is a database compiled and maintained
by EPA. It contains data on all PWSs including system location, system type (such as
community or non-community water systems), primary raw water source (ground water or
surface water), and violations. Optional reporting fields include type of treatment and ownership
type. Because providing some data is discretionary, EPA does not have complete data on every
system for these parameters. This is particularly common for non-community water systems
(NCWSs).
3-45

Drinking Water Industry Report Section 3 – Industry Profile
Because SDWIS is continuously being updated, EPA used 155,693 records of
active PWSs from the third quarter of 2007 for this economic profile (U.S. EPA, 2007).
3.3.1.2 Community Water System Survey
The second source of information, the CWSS, is a periodically updated detailed
EPA survey of surface and ground water community water systems (CWSs). The most recent
survey was conducted in 2000 and published in 2002 (U.S. EPA, 2002). See Section 2.3.2 for
more details. Since there is no survey equivalent to CWSS for non-community water systems,
the operational and financial information presented later in this profile is only available for
CWSs (U.S. EPA, 2002).
3.3.1.3 EPA DWT Industry Questionnaire
The EPA DWT Industry Questionnaire, conducted in 2007, is a survey of WTPs
specifically created for this study to gather data on the operation, financial characteristics, and
residuals discharges from the industry. The technical operations questions were posed at the
water treatment plant level. The financial portion of the survey (questions 4 through 13) asked
for system or utility level data depending on whether the costs for a treatment technology would
be spread amongst consumers at the system level or across all the customers of the larger utility.
For the purpose of determining the financial strength of the larger corporate entity which owns
the individual drinking water treatment plant being surveyed in the engineering portion of the
survey and the impacts to the large corporate entity’s customer base EPA must look to the level
of the system. It is at the system level that the costs of technology improvements are financed
and it is the system that can spread the costs of upgrades to a specific plant or plants across its
total customer base. In some instances a larger utility may own more than one system and
spreads the cost of technology improvements across those systems. In this case the proper level
of financial assessment is at the level of the utility.
10
See Section 2.2 for further details.
10
In the EPA DWT Industry Questionnaire respondents were instructed to give either system or utility level
information in their financial survey responses depending on which characterization was most appropriate. The
competed responses to the financial portion of the survey are all at the system level. They may also be referred to as
single system utility level data.
3-46

Drinking Water Industry Report Section 3 – Industry Profile
3.3.2 Public Water System Characteristics
As discussed in Section 3.1, there are two major types of PWSs: community and
non-community water systems (CWSs and NCWSs). This section discusses the different types of
PWSs and the major characteristics used to classify them. Basic characteristics such as
population served, ownership, and water source are discussed first, followed by operational
characteristics such as water treatment and residual management. The purpose of this section is
to provide a snapshot of the public water system industry. Table 3-25 provides a breakdown of
PWSs by system type, according to SDWIS.
Table 3-25. Number of PWSs and Total Population Served by System Type, SDWIS
System Type
Systems
Population Served
CWS
52,110
33%
286,451,204
93%
NCWS
103,583
67%
20,086,152
7%
Total
a
155,693
100%
306,537,356
100%
Source: U.S. EPA, 2007.
a – Four systems of an “unspecified” system type are included in these totals.
3.3.2.1 Population Served
Table 3-26 presents the number of systems by type and by the number of people
(as a range) served by each system, according to SDWIS. The table shows that the vast majority
of both community and non-community water systems are fairly small, serving a population of
less than 3,000 people. Only 8 percent of CWSs and 0.04 percent of NCWSs are large (serve
more than 10,000 people).
Table 3-26. Summary of the Number of PWSs by System Type and Size, SDWIS
System Type
System Size (Population Served)
<100
101 - 500
501 - 3k
3k - 10k
10k - 50k
>50k
Total
a
CWS
13,270
16,012
13,906
4,822
3,175
925
52,110
NCWS
71,170
26,737
5,413
222
33
8
103,583
Total
84,440
42,749
19,319
5,044
3,208
933
155,693
Source: U.S. EPA, 2007.
a – components may not add up to totals due to rounding.
3-47

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-27 shows the number of systems, by source water and population served ,
reporting water sales for each customer category. This data is from the EPA DWT Industry
Questionnaire; the total number of systems listed , 285, is a subset of the SDWIS systems
.
11
)
This table shows that 95 percent of systems serve residential customers, 89 percent of systems
serve non-residential customers, and 65 percent of systems sell water to other systems.
Table 3-27. Number of Systems that Report Water Sales to Different Customer Categories,
DWT Industry Questionnaire
Primary
Source*
Population Served
Sold to Other
Systems
Residential
Customers
Non-Residential
Customers
Other
Surface
10,000-50,000
48
73
67
59
More than 50,000
123
155
151
142
Ground
10,000-50,000
4
17
15
14
More than 50,000
10
25
21
22
Total
185
270
254
237
Note: Systems serve more than one customer type—totals are not of unique systems. All systems report at least one
customer type.
*Systems that use purchased water as their primary source are not presented in this table because of the potential for
revealing Confidential Business Information Source: Appendix A.
3.3.2.2 Ownership
PWSs are owned by a variety of public and private entities. Public PWSs may be
owned by a federal, state, or local entity, or by a Native American tribe. Private PWSs may be
owned by non-profit or for-profit firms, or may be operated as ancillary businesses to other
enterprises. Some PWSs are also co-owned by public and private entities.
Table 3-28 summarizes the number of PWSs by ownership type and size of the
population served, according to SDWIS. Public entities such as federal, state, and local
government agencies and Native American tribes own approximately 27 percent of all PWSs in
the U.S. Privately-owned PWSs make up approximately 69 percent of all PWSs. The majority of
privately-owned PWSs, however, are small with over 71 percent serving fewer than 10,000
people. They make up only 15 percent of PWSs serving over 50,000 people. In total, privately
11
285 is the number of completed system responses to the financial portion of the EPA DWT Industry
Questionnaire without those systems that primarily resell water that is purchased from other systems. The purchased
water source category has been omitted from the results presented in this section because of the potential for
revealing Confidential Business Information.
3-48

Drinking Water Industry Report Section 3 – Industry Profile
owned PWSs provide water to only 18 percent of the population served by PWSs, while
publicly-owned systems serve about 80 percent (U.S. EPA, 2007).
Table 3-28. Number of Water Systems by Ownership Type and Size, SDWIS
Type of Ownership
System Size (Population Served)
Population Served
<10k
10k - 50k
>50k
Total
Public
38,601
2,722
789
42,112
245,085,282
Federal Government
3,736
66
5
3,807
3,038,437
State Government
5,370
40
6
5,416
5,957,549
Local Government
28,560
2,604
778
31,942
235,112,533
Native American
935
12
0
673
976,763
Private
106,899
437
134
107,470
56,238,197
Mixed public/private
6,052
49
10
6,111
5,213,877
Total
151,552
3,208
933
155,693
306,537,356
Source: U.S. EPA, 2007.
Table 3-28 does not present systems according to the type of population served,
but groups these systems together. In general, a larger percentage of NCWSs than CWSs are
privately owned. Privately-owned PWSs account for approximately 82 percent of TNCWSs and
69 percent of NTNCWSs, as compared to only 48 percent of CWSs.
3.3.2.3 Water Source
In addition to the type and size of population served and the type of ownership,
water systems can be classified by their primary water source. PWSs may rely on ground water,
surface water, or water purchased from other water systems. Table 3-29 presents the number of
PWSs that draw water from each type of water source, by the size of the population served
according to SDWIS.
12
The table also presents the total number of people that receive water
from each type of water source. The vast majority of PWSs draw water from ground sources.
The percent of PWSs utilizing ground water decreases significantly, however, as the size of the
population served increases. The percentage of PWSs utilizing surface water, on the other hand,
increases with the increase in the population served. In total, about 92 percent of PWSs draw
water from ground sources. These systems, however, distribute water to only 36 percent of the
12
SDWIS classifies a water system as relying on surface water if any of its water comes from surface water sources.
3-49

Drinking Water Industry Report Section 3 – Industry Profile
total populations served by PWSs. Sixty-four percent of PWSs’ customers receive water drawn
from surface sources.
Table 3-29 does not present PWSs according to the type of population served, but
groups these systems together. PWSs that draw from ground water account for approximately 74
percent of CWSs, as compared to only 13 percent of NCWSs. PWSs that draw from surface
water account for approximately 97 percent of CWS, as compared to only 2 percent of NCWSs.
Table 3-29. Number of Water Systems by Water Source and System Size, SDWIS
Type of Source
Water
System Size (Population Served)
Population Served
<10k
10k - 50k
>50k
Total
Ground water
137,371
1,344
231
138,946
105,598,776
Surface water
4,043
938
455
5,436
137,577,368
Purchased
10,100
925
247
11,272
63,298,151
Ground water
3,669
60
7
3,736
4,676,746
Surface water
6,431
865
240
7,536
58,621,405
Total
a
151,552
3,208
933
155,693
306,537,356
Source: U.S. EPA, 2007.
a – Totals include 12 systems in the “< 10k” category that use an “unspecified” water type.
As identified in Table 3-27 within the EPA DWT Industry Questionnaire in the
10,000 to 50,000 population category, approximately 17 percent of the systems draw from
ground water and 83 percent draw from surface water. Within the greater than 50,000 population
category, approximately 14 percent of the respondent systems draw from ground water and 86
percent draw from surface water.
3.3.2.4 Operational Characteristics: Water Treatment and Direct Discharge to
Surface Water
This section presents CWSS data on two characteristics: water treatment and
residuals management. Because SDWIS does not provide data on either treatment practices or
residuals management, this information is not available for NCWSs. This section also does not
present the characteristics for systems surveyed by the EPA DWT Industry Questionnaire;
national estimates for WTPs are presented in Section 3.2.
3-50

Drinking Water Industry Report Section 3 – Industry Profile
• Water Treatment: Not all CWSs treat water prior to distributing it to their
customers. Some CWSs purchase water that has already been treated from
other drinking water systems while other CWSs draw their water from
sources that are pure enough to satisfy federal drinking water guidelines,
eliminating the need for treatment. Systems that do not treat water are
assumed not to discharge to surface water. CWSS asks respondents to
report whether or not they treat water and several detailed questions
regarding the treatment technology used. Overall, 75 percent of ground
water systems, 99.6 percent of surface water systems, and 17 percent of
systems purchasing water provide treatment (U.S. EPA, 2002).
• Residual Management: CWSs use a variety of technologies to dispose of
water treatment residuals such as sludge, sediment, and chemicals. Some
of the residual management techniques used by water systems include
mechanical dewatering, land application, deep well injection, and direct
discharge to surface water. Overall, 3 percent of ground water systems, 10
percent of surface water systems, and 6 percent of systems purchasing
water perform residuals treatment (U.S. EPA, 2002).
Table 3-30 presents the number of large CWSs (serving more than 10,000 people)
that provide treatment and the number of CWSs that discharge directly to surface water,
according to CWSS. The information is presented for all systems and by water source.
3-51

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-30. Summary of CWSs by Water Source and Population Served, CWSS
System Size (Population Served)
10k-50k
>50k
Total >10k
Ground Water
All Systems
1,340
307
1,647
Provide Treatment
983
233
1,216
Discharger
217
50
267
Surface Water
All Systems
988
440
1,428
Provide Treatment
977
434
1,411
Discharger
387
142
529
Purchased Water
All Systems
685
238
923
Provide Treatment
385
100
485
Discharger
27
19
46
Total
All Systems
3,013
985
3,998
Provide Treatment
2,345
767
3,112
Discharger
631
211
842
Source: U.S. EPA, 2002.
Table 3-31 presents the 2006 water quantity sold, in million gallons per year
(MGY), per system, reported at the 25
th
, 50
th
, and 75
th
quartiles, according to the EPA DWT
Industry Questionnaire responses. The median quantity sold in 2006 across all respondent
surface and ground water source systems was 4,297 million gallons.
3.3.3 Financial Characteristics of Drinking Water Treatment Systems
In order to gauge the ability of PWSs to comply with environmental regulations,
EPA conducts analyses that assess the financial health of the industry. This section provides a
snapshot of the financial state of large CWSs (serving over 10,000 people).
Basic data on revenue, expenses, capital expenditures, and funding sources
available to water systems was obtained from CWSS and responses to the EPA DWT Industry
Questionnaire questions 4 through 13. Because SDWIS does not provide any data on finances of
the encompassed systems, no such information was available for non-community systems. This
3-52

Drinking Water Industry Report Section 3 – Industry Profile
section first presents system revenues and revenue sources, followed by system expenses and
funding availability.
3.3.3.1 Water System Revenues
Water sales are the primary source of revenue for the vast majority of water
systems.
13
CWSs supply water to private homes, businesses, agricultural and other non-
residential customers. A portion of CWS revenues also comes from connection fees, inspections,
penalties and fines, and other non-consumption based charges.
Total CWS revenues came to $39 billion in 2000 (2000$). Revenues of publicly-
owned systems accounted for 88 percent of this total. Water sales revenues contributed $33
billion (85 percent) of total CWS revenues, and residential water sales accounted for about 60
percent of total water sales for CWSs of all sizes. Overall, residential revenues have increased
slightly since 1995 (U.S. EPA, 2002).
Table 3-32 presents the 25
th
percentile. median, and 75
th
percentile values for
revenue by ownership type and system size, according to CWSS. The table shows that private
systems earn slightly higher revenues than public systems.
13
Although some smaller systems may be run as ancillary businesses, this was not true for any of the systems with a
population of greater than 10,000 served.
3-53

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-31. Reported 2006 Water Quantity Sold (MGY), per System, DWT Industry Questionnaire
Primary Source
a
Population Group
Number of
Systems
Water Quantity Sold (MGY)
25th Percentile
50th Percentile
75th Percentile
Surface
10,000-50,000
74
657
1,351
2,274
More than 50,000
166
4,403
8,488
17,333
Ground
10,000-50,000
18
594
758
1,435
More than 50,000
27
2,026
3,700
5,871
Total Systems/Quantity Across All Categories
285
1,664
4,297
11,242
Source: Appendix A.
a – Systems that use purchased water as their primary source are not presented in this table because of the potential for revealing Confidential Business
Information.
Table 3-32. Summary of Annual CWS Revenues by Ownership Type ($1,000), CWSS
System Size
(Population Served)
Ownership Type
Public
Private
All Systems
P25
P50
P75
P25
P50
P75
P25
P50
P75
10k-50k
$1,566
$2,302
$3,373
$1,454
$2,465
$4,100
$1,566
$2,313
$3,386
50k-100k
$5,344
$7,126
$11,254
$8,086
$10,133
$14,830
$5,440
$7,313
$11,802
100k-500k
$9,674
$16,444
$27,767
$15,217
$15,970
$36,579
$9,885
$16,187
$27,811
>500k
$61,899
$89,897
$193,345
$121,339
$122,075
$171,568
$62,103
$99,807
$188,013
Source: U.S. EPA, 2002.
P25 – 25
th
percentile.
P50 – 50
th
percentile (median).
P75 – 75the percentile.
3-54

Drinking Water Industry Report
S
Table 3-33 presents the median revenue per 1,000 gallons (and the 25
th
and 75
th
percentiles), also by ownership type and system size, for those CWSs identified as discharging,
according to CWSS. Similar to annual revenue, private systems also earn significantly more per
gallon than their public counterparts. This discrepancy decreases with system size. For both private
and public systems, revenue per 1,000 gallons generally declines as system size grows.
Table 3-33. Summary of Total Revenues of CWSs that Discharge ($/1,000 gallons)
System Size
(Population Served)
Ownership Type
Public
Private
All Systems
P25
P50
P75
P25
P50
P75
P25
P50
P75
10k-50k
$1.40
$1.93
$2.83
$3.16
$3.26
$3.36
$1.41
$2.51
$3.07
50k-100k
$1.12
$1.58
$1.71
$0.93
$2.42
$4.24
$1.12
$1.71
$1.74
100k-500k
$1.36
$1.82
$2.24
$2.17
$2.18
$2.58
$1.45
$1.95
$2.25
>500k
$1.37
$1.69
$1.71
N/A
N/A
N/A
$1.37
$1.69
$1.71
Source: U.S. EPA, 2002.
P25 – 25
th
percentile.
P50 – 50
th
percentile (median).
P75 – 75the percentile.
Table 3-34 presents the 2006 total revenue per system (in millions), reported at the
25
th
, 50
th
, and 75
th
quartiles, according to responses to the EPA DWT Industry Questionnaire.
Median total annual revenue across all source water and population size categories was $14 million.
Surface source water systems serving both populations between 10,000 and 50,000, and those
serving greater than 50,000 people reported higher median revenues, $4.5 and $25.3 million
respectively, than their ground water counterparts. Table 3-35 presents 2006 revenues per volume
(dollars per million gallons) from the EPA questionnaire. Unlike the total revenue values in Table 3-
34 the per unit water sales median values show that ground water systems receive higher per unit
revenues than surface water systems. Ground water systems serving between 10,000 and 50,000
people sell water at a median price of $4,021 per million gallons while surface water systems serving
the same number of people receive a median sale value of $3,379. Systems serving more people and
dealing in greater amounts of delivered water generally sell water at lower per unit prices. The
median 2006 revenue per million gallons across all systems that responded to the DWT Industry
Questionnaire was $3,082.
3-55

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-34. Reported 2006 Revenues by Population Served and Primary Water Source, per System, DWT Industry
Questionnaire
Primary Source
a
Population Group
Number of
Systems
Revenues ($ Millions)
25th Percentile
50th Percentile
75th Percentile
Surface
10,000-50,000
74
$3.2
$4.5
$6.8
More than 50,000
166
$13.8
$25.3
$49.5
Ground
10,000-50,000
18
$2.4
$3.2
$3.8
More than 50,000
27
$9.9
$14.9
$23.3
Total Systems/ Revenues Across All Categories
285
$6.2
$14
$32.7
Source: Appendix A.
a – Systems that use purchased water as their primary source are not presented in this table because of the potential for revealing Confidential Business
Information.
Table 3-35. Reported 2006 Water Sales Revenue per Volume, per System, DWT Industry Questionnaire
Primary
Source
a
Population Group
Number of
Systems
Water Sales Revenue per Volume ($/MGY)
25th Percentile
50th Percentile
75th Percentile
Surface
10,000-50,000
74
$2,230
$3,379
$5,216
More than 50,000
166
$2,043
$2,826
$3,765
Ground
10,000-50,000
18
$2,202
$4,021
$5,893
More than 50,000
27
$2,655
$3,867
$6,723
Total Systems/ Sales Revenue Across All Categories
285
$2,199
$3,082
$4,599
Source: Appendix A.
a – Systems that use purchased water as their primary source are not presented in this table because of the potential for revealing Confidential Business
Information.
3-56

Drinking Water Industry Report Section 3 – Industry Profile
3.3.3.2 Expenses
CWSs spent a total of $32 billion in 2000 on routine operating expenses,
including water treatment, water distribution, and residuals management. Expenses of systems
with a population of greater than 10,000 served totaled $13.3 billion (U.S. EPA, 2002).
According to CWSS employee compensation – including salary, benefits, and
contractor payments – accounts for about 31 percent of total system expenditures. Other routine
operating and maintenance expenses account for another 45 percent. In total, operating expenses
(employee expenses and other operating and maintenance expenditures) account for about 75
percent of total system expenditures. Debt service payments and other expenses, contribute
another 19 percent (U.S. EPA, 2002).
Table 3-36 presents average total system expenditures by ownership type and
system size, according to CWSS. The table also presents a breakdown of expenses by major
category (employee, routine operating, debt service expenditures, and other expenses).
3-57

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-36. Average System Expenses and Expense Breakdown by Major Category, CWSS
System Size (Population Served)
10k- 50k
50k-100k
100k –500k
> 500k
Total
Across All
a
All Systems
Average System Expenses ($000)
$2,673
$7,617
$18,561
$129,320
$7,539
Employeeb
28%
30%
32%
34%
31%
Routine Operating
70%
62%
52%
48%
45%
Debt Service
2%
6%
12%
14%
19%
Other Expenses
1%
3%
4%
5%
6%
Public Systems
Average System Expenses ($000)
$2,675
$7,630
$18,408
$131,490
$7,805
Employeeb
34%
32%
32%
33%
30%
Routine Operating
45%
52%
52%
46%
44%
Debt Service
16%
11%
12%
16%
20%
Other Expenses
5%
6%
3%
5%
6%
Private Systems
Average System Expenses ($000)
$2,664
$7,470
$20,466
$94,419
$5,355
Employeeb
28%
29%
32%
40%
34%
Routine Operating
72%
69%
53%
56%
51%
Debt Service
1%
3%
10%
4%
9%
Other Expenses
1%
1%
6%
3%
8%
Source: U.S. EPA, 2002.
a – Components may not add up to 100% due to rounding.
b – Employee expenses include contractor expenses.
Table 3-37 presents the median and bounding quartiles for total expenses per
1,000 gallons of water produced by ownership type and system size for CWSs, according to
CWSS.
Table 3-37. Summary of Total Expenses by System Size and Ownership Type ($/1,000
gallons produced), CWSS
System Size
(Population Served)
Ownership Type
Public
Private
Across All Systems
P25
P50
P75
P25
P50
P75
P25
P50
P75
10k-50k
$1.27
$2.05
$2.95
$1.99
$2.30
$2.68
$1.29
$2.11
$2.79
50k-100k
$1.16
$1.67
$2.32
$1.66
$2.11
$2.99
$1.16
$1.67
$2.41
100k-500k
$1.20
$1.93
$2.48
$1.42
$2.09
$2.85
$1.22
$1.93
$2.48
>500k
$1.09
$1.70
$2.06
$1.49
$2.05
$2.17
$1.21
$1.71
$2.10
Source: U.S. EPA, 2002.
P25 – 25
th
percentile. P50 – 50
th
percentile (median). P75 – 75the percentile.
3-58

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-38, Table 3-39, and Table 3-40 report the 25
th
, 50
th
, and 75
th
quartiles for
2006 total expenses per system, total and routine operating expenses per million gallons a year
for each system, and total employee wages per system, respectively from the DWT Industry
Questionnaire.
Based on the EPA DWT Industry Questionnaire responses representing 285
systems, median total annual expenses equaled $15.6 million. Seventy-five percent of systems
reported total costs of operation below $39.6 million. Median total expenses per million gallons
of produced water ranged from $3,272 to $4,815 across the source water and population served
categories. Routine per unit operating expenses were highest for ground water systems serving
more than 50,000 people, at $3,268. Surface water systems serving more than 50,000 people had
the lowest per unit routine expenditures, $1,897. Across all respondent categories median total
per unit expenses equaled $3,522. Median routine operating expenses across all respondents was
$2,034 or about 58% of the total median expenditures value.
Table 3-40 shows that the median hourly wage rate paid in 2006 among EPA’s
survey responders equals $26. The median hourly wage rate ranged from $23 an hour paid by
surface water producers serving between 10,000 and 50,000 residents, and $27 an hour paid by
surface water suppliers serving greater than 50,000 people. Median total annual wages paid by
the surveyed drinking water systems in 2006 was $1,447,000. Fifty percent of the 251
respondents to this question paid between $616,000 and $3,535,000 in wages for the year 2006.
3-59

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-38. Reported 2006 Total Expenses, per System, DWT Industry Questionnaire
Source: Appendix A.
a – Systems that use purchased water as their primary source are not presented in this table because of the potential for revealing Confidential Business
Information.
Primary Source
a
Population Group
Number of
Systems
Total Expenses ($in millions)
25th Percentile
50th Percentile
75th Percentile
Surface
10,000-50,000
74
$2.8
$4.7
$7.5
More than 50,000
166
$14.5
$30.2
$58.1
Ground
10,000-50,000
18
$2.0
$3.0
$4.6
More than 50,000
27
$12.0
$16.8
$36.3
Total Systems/ Expenses Across All Categories
285
$6.4
$15.6
$39.6
3-60

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-39. Reported 2006 Expenses per MGY, Total and Operating, per System, DWT Industry Questionnaire
Source: Appendix A.
a – Systems that use purchased water as their primary source are not presented in this table because of the potential for revealing Confidential Business
Information
Primary
Source
a
Population Group
Number of
Systems
Total Expenses ($/MGY)
Routine Operating Expenses ($/MGY)
25th Percentile
50th Percentile
75th Percentile
25th Percentile
50th Percentile
75th Percentile
Surface
10,000-50,000
74
$2,186
$3,272
$5,653
$1,342
$2,105
$3,244
More than 50,000
166
$2,377
$3,406
$4,906
$1,324
$1,897
$2,847
Ground
10,000-50,000
18
$2,196
$3,900
$6,762
$1,267
$2,516
$3,407
More than 50,000
27
$3,231
$4,815
$10,489
$2,046
$3,268
$4,810
Total Systems/ Expenses
Across All Categories
285 $2,357 $3,522 $5,474 $1,378 $2,034 $3,186
3-61

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-40. Reported 2006 Hourly and Total Wages for All Employees, per System, DWT Industry Questionnaire
Primary
Source
a
Population
Group
Number of
Systems
b
Number of
Employees
Hourly Wage ($)
Total Wages Annually ($ in thousands)
25th
Percentile
50th
Percentile
75th
Percentile
25th
Percentile
50th
Percentile
75th
Percentile
Surface
10,000-50,000
69
1,249
$20
$23
$26
$398
$579
$1,088
More than 50,000
139
15,433
$22
$27
$33
$1,441
$2,694
$5,142
Ground
10,000-50,000
18
249
$19
$25
$27
$323
$543
$645
More than 50,000
26
1,126
$23
$26
$30
$765
$1,454
$1,927
Total Systems/ Wages
Across All Categories
251
18,579
$22
$26
$30
$616
$1,447
$3,535
Source: Appendix A.
a – Systems that use purchased water as their primary source are not presented in this table because of the potential for revealing Confidential Business
Information.
b – There is a smaller number of systems in this table as some systems did not report wages, or reported them in an unclear manner.
3-62

Drinking Water Industry Report Section 3 – Industry Profile
3.3.4 Customer Profile
Most CWSs, especially the ones serving the larger populations, are expected to be
able to pass on any technology costs to their customers through rate increases. As a result, it is
important to conduct an assessment of the likely burden on households served by CWSs.
This section provides information on the customers of regulated CWSs, including
customer types and water deliveries, revenues, and water rates by customer type. For residential
customers, this section also discusses average annual water bills per household, information on
the billing structures utilized, the availability of subsidized rates for low income families, and the
average annual income of households served by regulated CWSs.
3.3.4.1 Customer Types
CWSs serve three primary customer types: (1) other water suppliers, who resell
water to the final customers, (2) residential customers, and (3) non-residential customers. Non-
residential customers can be further divided into commercial, industrial, agricultural, and other
customers (e.g., hospitals and schools, prisons, or governments).
According to the CWSS, of the systems with a population of greater than 10,000
served, 1,680 (or 43 percent) sell water to other water suppliers, 3,242 (or 83 percent) serve
residential customers, and 3,024 (or 77 percent) serve non-residential customers. Of the systems
that serve non-residential customers, 91 percent serve commercial/industrial customers, 10
percent serve agricultural customers, and 32 percent serve other non-residential customers. Table
3-41 presents the number and percentage of systems with a population of greater than 10,000 that
serve the different types of customers, by system size, according to CWSS. The majority of all
systems, irrespective of size, serve residential and non-residential customers, while the largest
systems (serving 500,000 people and more) are more likely to sell water to other water systems
than the smaller-sized systems.
3-63

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-41. Number and Percentage of CWSs Serving Different Customer Types, CWSS
System Size (Population Served)
10k - 50k
50k - 100k
100k - 500k
> 500k
Total
a
Sold to Other PWS
1,107
37%
247
53%
251
60%
75
88%
1,680
43%
Residential
2,478
84%
384
82%
314
75%
66
78%
3,242
83%
Non-Residential
2,282
77%
371
79%
302
72%
69
82%
3,024
77%
Commercial/Industrial
2,112
93%
346
93%
232
77%
52
75%
2,742
91%
Agricultural
214
9%
38
10%
48
16%
9
12%
309
10%
Other
603
26%
143
39%
173
57%
37
53%
956
32%
Total
2,952
100%
470
100%
421
100%
85
100%
3,928
100%
Source: U.S. EPA, 2002.
a – Fifteen of the 2,283 systems with a population of greater than 10,000 served are excluded from these numbers
because of removal of outliers in the CWSS data.
3.3.4.2 Water Deliveries, Revenues, and Rates by Customer Type
In 2000, the CWSs with a population of greater than 10,000 served supplied over
17,317 billion gallons of water to their customers.
14
Thirty-eight percent of this amount was
delivered to residential customers, 22 percent was delivered to non-residential customers, 23
percent was sold to another CWS, and 7 percent of the water was unaccounted for. Systems
serving over 500,000 people accounted for the largest share of total water deliveries, with 40
percent, followed by systems serving 100,000 to 500,000 people, with 26 percent.
Table 3-42 presents year 2000 water deliveries by population served and customer
type, according to CWSS. The table also distinguishes between systems owned by private and
public entities. Based on ownership type, private systems deliver 49 percent of all water to
residential customers, compared to 37 percent for public systems. This difference is especially
pronounced in the largest size category (more than 500,000 people served). Conversely, public
systems deliver 25 percent of their water to other CWSs, compared to only 6 percent for private
systems.
14
These numbers are based on 2,268 of the 2,283 regulated systems, that provided information on water deliveries.
3-64

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-42. Amount of Water Delivered by Customer and Ownership Type and System
Size (billion gallons; 2000), CWSS
System Size (Population Served)
10k - 50k
50k - 100k
100k - 500k
> 500k
Total
All Systems
Sold to Other PWS
468
12%
180
9%
638
14%
2,774
40%
4,061
23%
Residential
1,700
43%
878
45%
1,762
40%
2,191
31%
6,530
38%
Non-Residential
893
23%
542
28%
1,004
23%
1,377
20%
3,816
22%
Unaccounted for
290
7%
187
10%
320
7%
480
7%
1,278
7%
Totala
3,930
100%
1,960
100%
4,436
100%
6,991
100%
17,317
100%
Public Systems
Sold to Other PWS
443
13%
165
9%
610
15%
2,759
42%
3,976
25%
Residential
1,440
42%
762
43%
1,616
40%
1,989
30%
5,807
37%
Non-Residential
832
24%
487
28%
899
22%
1,313
20%
3,531
22%
Unaccounted for
245
7%
166
9%
283
7%
461
7%
1,155
7%
Totala
3,402
100%
1,753
100%
4,072
100%
6,623
100%
15,850
100%
Private Systems
Sold to Other PWS
25
5%
16
8%
29
8%
15
4%
85
6%
Residential
260
49%
116
56%
146
40%
202
55%
724
49%
Non-Residential
61
11%
54
26%
105
29%
64
18%
285
19%
Unaccounted for
45
9%
21
10%
37
10%
19
5%
122
8%
Totala
529
100%
207
100%
364
100%
367
100%
1,467
100%
Source: U.S. EPA, 2002.
a – Sum of individual components may not add up to total due to missing data in some of the subaccounts.
Table 3-43 presents the 25
th
percentile, median, and 75
th
percentile values for
water sales for residential customers, in millions of gallons per year, according to the EPA DWT
Industry Questionnaire respondents.
3-65

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-43. Reported 2006 Water Sales to Residential Customers, by System, DWT Industry Questionnaire
Primary
Source
a
Population Group
Number of
Systems
b
Estimated Water Sold (MGY)
25th Percentile
50th Percentile
75th Percentile
Surface
10,000-50,000
73
332
654
1,090
More than 50,000
155
2,094
3,800
7,889
Ground
10,000-50,000
17
273
331
484
More than 50,000
25
1,601
2,819
4,113
Total
270
740
2,187
4,933
Source: Appendix A.
a – Systems that use purchased water as their primary source are not presented in this table because of the potential for revealing Confidential Business
Information.
b – There are fewer systems in this table than in the others as only systems with residential sales are reported.
3-66

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-44 presents 2000 water sales revenue of CWSs, by population served and
customer type, according to CWSS.
15
The table also distinguishes between systems owned by
private and public entities. In 2000, these large CWSs received $27.2 billion in water sales
revenue. Public systems accounted for almost 89 percent of this total. Similar to water deliveries
discussed above, residential customers account for the largest share of water sales revenues.
However, while residential customers accounted for 38 percent of water deliveries in 2000, they
accounted for 48 percent of revenues, indicating higher average rates for this customer group.
Table 3-44. Revenues by Customer Type (in million $), CWSS
10k - 50k
50k - 100k
100k - 500k
> 500k
Total
All Systems
Sold to Other PWS
$403
6%
$212
7%
$852
12%
$2,922
29%
$4,388
16%
Residential
$3,796
57%
$1,795
55%
$3,719
52%
$3,894
38%
$13,204
48%
Non-Residential
$1,601
24%
$761
23%
$1,973
28%
$2,348
23%
$6,683
25%
Totala
$6,715
100%
$3,250
100%
$7,150
100%
$10,127
100%
$27,242
100%
Public Systems
Sold to Other PWS
$351
6%
$192
7%
$822
13%
$2,911
32%
$4,277
18%
Residential
$3,235
56%
$1,506
53%
$3,283
51%
$3,389
37%
$11,413
47%
Non-Residential
$1,525
26%
$694
24%
$1,715
27%
$2,148
23%
$6,082
25%
Totala
$5,774
100%
$2,840
100%
$6,411
100%
$9,176
100%
$24,202
100%
Private Systems
Sold to Other PWS
$51
5%
$20
5%
$30
4%
$10
1%
$111
4%
Residential
$561
60%
$289
71%
$436
59%
$504
53%
$1,790
59%
Non-Residential
$76
8%
$67
16%
$258
35%
$199
21%
$601
20%
Totala
$941
100%
$409
100%
$738
100%
$951
100%
$3,040
100%
Source: U.S. EPA, 2002.
a – Sum of individual components may not add up to total due to missing data in some of the subaccounts.
Table 3-45 presents median revenue (per 1,000 gallons of water delivered) of
CWSs, by population served and customer type, according to CWSS.
16
Similar to Table 3-44
above, Table 3-45 also distinguishes between systems owned by private and public entities. The
table shows that non-residential customers served by privately-owned CWSs have the highest
15
These numbers are based on 2,063 of the 2,283 regulated systems that provided information on water sales
revenues.
16
These numbers are based on 2,014 of the 2,283 regulated systems that provided information on water sales
revenues and water deliveries.
3-67

Drinking Water Industry Report Section 3 – Industry Profile
median water rates of $4.27 per 1,000 gallons. For all customer groups, private systems charge
higher rates than public systems.
Table 3-45. Median Revenue per 1000 Gallons of Water Delivered by Customer Type,
Ownership Type, and System Size ($/1000 gallons), CWSS
System Size (Population Served)
10k-50k
50k-100k
100k-500k
>500k
Total
All Systems
Sold to Other PWS
$1.54
$1.61
$1.09
$1.09
$1.57
Residential
$2.80
$2.61
$2.05
$1.89
$3.20
Non-Residential
$2.10
$2.14
$1.86
$1.74
$1.75
Total
a
$2.02
$1.65
$1.79
$1.62
$2.73
Public Systems
Sold to Other PWS
$1.32
$1.43
$1.09
$1.02
$1.57
Residential
$2.72
$2.49
$2.02
$1.85
$3.03
Non-Residential
$2.09
$1.93
$1.79
$1.66
$1.73
Total
a
$1.86
$1.61
$1.73
$1.56
$2.48
Private Systems
Sold to Other PWS
Residential
$2.30
$2.87
$2.00
$3.45
$1.28
$3.26
$1.16
$2.60
$1.44
$3.55
Non-Residential
$2.96
$2.91
$2.36
$3.06
$4.27
Total
a
$2.47
$3.09
$2.47
$2.66
$3.22
Source: U.S. EPA, 2002.
a – Total quantity (denominator) includes unaccounted for water for which no revenues were received.
3.3.4.3 Households
CWSs derive approximately 50 percent of their water sales revenue from
residential customers, with smaller CWSs depending more heavily on this customer class than
the larger CWSs. The average annual residential water bill for systems of every size, ownership
type, and water source category is $266 in the year 2000. Based on this average annual
residential water bill, and the national median household income of $42,151, most households
spend less than 1 percent of their annual income on water services (U.S. EPA, 2002).
Table 3-46 presents the median annual water bill for systems with a population of
greater than 10,000 served, by system size, ownership type, and water source, according to the
CWSS. In general, the median annual residential water bill is higher for privately-owned systems
than for publicly-owned systems, at $280 and $240, respectively. Additionally, across all
population size categories systems using ground water have the lowest median annual water
bills, followed closely by surface water systems, with purchased water systems having
3-68

Drinking Water Industry Report Section 3 – Industry Profile
significantly higher median annual residential water bills. These generalizations do not hold for
the 50,000 to 100,000 and the greater than 500,000 categories where surface water systems have
lower median residential water bills than ground water systems.
Table 3-46. Summary of Median Annual Residential Water Bill, CWSS
System Size (Population Served)
10k-50k
50k-100k
100k-500k
>500k
Total
Overall
$269
$267
$224
$236
$266
By Ownership Type
Public
$240
$260
$211
$223
$240
Private
$260
$395
$350
$350
$280
By Water Source
Ground Water
$211
$262
$144
$251
$211
Surface Water
$264
$240
$234
$211
$249
Purchased Water
$360
$300
$299
$255
$338
Source: U.S. EPA, 2002.
Table 3-47 shows the estimated number of systems using various billing methods
for all customers, according to the EPA DWT Industry Questionnaire. Seventy-five percent of
systems use rates based on metered water usage. Approximately 50 percent of the systems that
reported a billing method have uniform rates. Peak seasonal rates are not common. Smaller
systems are more likely to use declining block rates than increasing block rates. As for larger
systems serving more than 50,000 people the story is mixed with surface water systems tending
towards declining block rates and ground water systems strongly skewed to the use of increasing
block rates.
Some of the variance in median annual residential water bills may be attributed to
the fact that some CWSs provide reduced rates to low- and fixed-income households (i.e.,
lifeline rates). Table 3-48 presents the number and percentage of systems that offer reduced rates
to low- and fixed-income households, according to CWSS. Overall, 3 percent of CWSs offer
reduced rates to qualifying household, while 69 percent do not (28 percent did not provide this
information). By ownership type, publicly-owned systems are more likely to offer lifeline rates
than privately-owned CWSs: 1,503 of 25,510 publicly-owned systems (6 percent) offer reduced
rates compared to only 18 of 26,675 privately-owned systems.
3-69

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-47. Number of Systems Using Various Billing Methods for All Customers, 2006, DWT Industry Questionnaire
Primary
Source
a
Population
Group
Metered Charges
Unmetered Charges
Other
Declining
Block rate
Increasing
Block Rate
Peak
Season
Rate
Uniform
Rate
Total
Metered
Annual
Connection
Fee
Combined
Flat Fee for
Water and
Other
Services
Separate
Flat F
ee for
Water
Total
Unmetered
Surface
10,000-50,000
26
20
2
36
84
5
2
23
30
10
More than
50,000
58 50 9 93 210 8 1 35 44 32
Ground
10,000-50,000
7
4
0
10
21
0
1
6
7
2
More than
50,000
5 17 3 13 38 2 0 6 8 2
Total
96
91
14
152
353
15
4
70
89
46
Source: Appendix A.
Note: Systems utilize more than one billing method—totals are not of unique systems. All systems report at least one billing method.
a – Systems that use purchased water as their primary source are not presented in this table because of the potential for revealing Confidential Business
Information.
3-70

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-48. Number and Percentage of Systems with Lower Rates for Low- or Fixed-
Income Households, CWSS
System Size (Population Served)
10k - 50k
50k - 100k
100k - 500k
> 500k
Total
No.
%
No.
%
No.
%
No.
%
No.
%
All Systems
Lower Rates Available
244
8%
43
9%
30
7%
18
22%
1,521
3%
Lower Rates Not
Available
2,552
85%
377
80%
360
84%
49
58%
35,958
69%
Did Not Report
217
7%
50
11%
41
9%
17
20%
14,707
28%
Total
3,013
100%
470
100%
430
100%
85
100%
52,186
100%
Public Systems
Lower Rates Available
232
9%
40
10%
30
8%
16
20%
1,503
6%
Lower Rates Not
Available
2,144
84%
328
79%
326
83%
45
59%
19,563
77%
Did Not Report
166
7%
47
11%
37
9%
16
21%
4,444
17%
Total
2,542
100%
415
100%
393
100%
76
100%
25,510
100%
Private Systems
Lower Rates Available
12
3%
3
6%
-
0%
3
33%
18
0%
Lower Rates Not
Available
408
87%
49
89%
34
91%
4
51%
16,395
61%
Did Not Report
51
11%
3
6%
3
9%
1
16%
10,263
38%
Total
471
100%
55
100%
37
100%
8
100%
26,675
100%
Source: U.S. EPA, 2002.
Table 3-49 shows the number of systems in 2006 that had a low income assistance
program, the 25
th
, 50
th
, and 75
th
quartiles for the number of low-income households qualifying
for the program, and the mean highest annual qualifying income for these programs (if the
system supplied the number), according to the EPA DWT Industry Questionnaire respondents.
Nearly 13 percent of systems offer some type of assistance program.
As shown in Table 3-50 according to the CWSS data, approximately 411,000
households were eligible for reduced rates in 2000, with qualifying household incomes ranging
from $0 to $54,000. Table 3-50 summarizes the number of households with reduced rates and the
qualifying income ranges by system size and ownership type. The table shows that most of the
households that qualify for the reduced rates receive their water from CWSs that serve greater
than 500,000 people or 10,000 to 50,000 people, with 189,770 households and 56,962
households in each group, respectively.
3-71

Drinking Water Industry Report Section 3 – Industry Profile
Table 3-49. Reported 2006 Household Participation in System Assistance Programs and Income Requirements, DWT Industry
Questionnaire
Source: Appendix A.
Note: Non-responses to this question were assumed to indicate that the system had no assistance program.
a – Systems that use purchased water as their primary source are not presented in this table because of the potential for revealing Confidential Business
Information.
Table 3-50. Number of Households with Lower Rates and Range of Qualifying Household Incomes, CWSS
System Size (Population Served)
10k - 50k
50k - 100k
100k - 500k
> 500k
Total
# of
Households
Min. - Max.
Inccome
# of
Households
Min. - Max.
Inccome
# of
Households
Min. - Max.
Inccome
# of
Households
Min. - Max.
Inccome
# of
Households
Min. - Max.
Inccome
Public
56,962
$0-29k
26,985
$10-54k
41,959
$15-33k
189,770
$17-29k
411,155
$0-54k
Private
n/a
n/a
n/a
n/a
n/a
n/a
a
a
a
a
All
Systems
56,962
$0-29k
26,985
$10-54k
41,959
$15-33k
189,770
$17-29k
411,155
$0-54k
Source: U.S. EPA, 2002.
a – Data not provided.
Primary
Source
a
Population Group
Number of Households
Highest Annual Income Requirement
Number of
Systems
25th
Percentile
50th
Percentile
75th
Percentile
Number of
Systems
Median (50th Percentile)
Surface
10,000-50,000
8
75
291
1,000
1
$25,000
More than 50,000
24
467
1,500
3,100
11
$35,000
Ground
10,000-50,000
1
9
9
9
0
NA
More than 50,000
3
146
150
779
2
$18,800
Total
36
148
704
2,567
14
$25,200
3-72

Drinking Water Industry Report Section 3 – Industry Profile
3.4 REFERENCES
Eastern Research Group (ERG), 2005. Memorandum: Review of Wholesale Drinking Water
Treatment Systems, Chantilly, VA. August 1, 2005. Document Control Number (DCN)
DW03783.
ERG, 2006. Memorandum: Drinking Water Treatment Residuals as RCRA Hazardous Waste.
Chantilly, VA. April 14, 2006. DCN DW00288.
U.S. EPA, 2002. Community Water System Survey 2000 (EPA 815-R-02-005), Office of Water,
Washington, DC. DCN DW00001.
U.S. EPA, 2006. SDWIS Inventory 2006-11-09 (MS Excel® file), Office of Water, November 9,
2006. DCN DW03717.
U.S. EPA, 2007. SDWIS Inventory (third quarter 2007) (MS Excel® file), Office of Water,
Washington, DC.
U.S. EPA, 2008a. FACTOIDS: Drinking Water and Ground Water Statistics for 2006 (EPA 816-
K-06-012). Office of Water, Washington, DC, March 2008. DCN DW03755.
3-73

SECTION 4
CURRENT STATE NPDES PERMIT REQUIREMENTS FOR
WATER TREATMENT PLANT RESIDUALS
This section presents the current wastewater discharge requirements for drinking
water treatment plant (WTP) residuals. Currently, there are no national effluent limitation
guidelines and standards, direct or indirect, to regulate discharges of residuals to waters of the
United States. Therefore, regulation of pollutants being discharged in residuals is decided by the
state (or other permitting authority) for direct dischargers and by the publicly-owned treatment
works (POTW) for indirect dischargers. Topics discussed in this section include an overview of
the state and federal National Pollutant Discharge Elimination System (NPDES) permit program
for WTPs (Section 4.1) and a summary of current pollutant limitations in NPDES permits for
WTPs (Section 4.2).
4.1 OVERVIEW OF STATE AND FEDERAL NPDES REGULATORY
REQUIREMENTS FOR WATER TREATMENT PLANTS
The NPDES permit program regulates residuals discharged directly to waters of
the United States. The permits are issued by EPA regional offices or authorized states
(permitting authority). WTPs may be authorized to discharge process wastewater (i.e., residuals)
under an individual or general NPDES permit. Individual NPDES permits are developed and
issued on a site-specific basis to manage the discharges at individual plants. General NPDES
permits are developed and issued for multiple plants with similar activities or effluent
characteristics. For both permit types, states apply water quality-based pollutant limitations
where required and develop technology-based best professional judgment (BPJ) limitations for
other pollutants.
EPA reviewed the 2004 Permit Compliance System (PCS) database to determine
how states are permitting discharges from WTPs. As discussed in Section 2.4.2, larger facilities
are more likely to appear in the PCS system as they are expected to impact surface waters to a
greater extent. Information on smaller facilities with less likelihood to impact surface waters is
4-1

Drinking Water Industry Report Section 4 – Current State NPDES Permit Requirements
not consistently tracked in PCS. Also, information might not be available for facilities with
discharges covered under a general permit.
The 2004 PCS database included 20 WTPs identified as major dischargers and
2,806 WTPs identified as minor dischargers. Of the 2,826 permit identification numbers in PCS,
971 WTPs (34%) have general permits and 1,855 WTPs (66%) have individual permits (ERG,
2005). Table 4-1 presents a summary of the PCS database
(http://www.epa.gov/enviro/html/pcs/pcs_subj.html) review by state.
Table 4-1. Summary of Permit Information in the 2004 Permit Compliance System
State
WTPs in EPA’s Permit
Compliance System
a
General Permits for WTP Discharges
General
Permits
Individual
Permits
Alabama
0
85
No general permit.
Alaska
0
3
No general permit.
Arizona
0
11
No general permit.
Arkansas
107
9
ARG640000: Water Treatment Plants
California
None listed
in PCS
33
Desalination concentrates covered under:
CAG9930001: Dewatering and Other Low Threat Discharges
(Central Coast Region)
Colorado
72 (COG64)
4 (COG38)
10
COG640000: General Permit for Water Treatment Plants
COG380000: Treated Water Distribution Systems
Connecticut
None listed
in PCS
1
GP-002: General Permit for the Discharge of Water Treatment
Wastewater Into Waters of the State of Connecticut
Delaware
0
None listed in
PCS
No general permit.
DC (Region 3)
0
1
No general permit.
Florida
1 (FLG07)
b
32
No general permit.
Georgia
0
1
No general permit.
Hawaii
0
1
No general permit.
Idaho (Region
10)
0
10
No general permit.
Indiana
0
101
No general permit.
Illinois
48
175
ILG640000: General Permit for Public Water Supply
Wastewaters
Iowa
0
14
No general permit.
Kansas
0
9
No general permit.
Kentucky
138
(KYG64)
2 (KYG20)
b
15
KYG64: General Permit for Wastewater Discharges Associated
with Drinking Water Plant Activities
4-2

Drinking Water Industry Report Section 4 – Current State NPDES Permit Requirements
Table 4-1. Summary of Permit Information in the 2004 Permit Compliance System
State
WTPs in EPA’s Permit
Compliance System
a
General Permits for WTP Discharges
General
Permits
Individual
Permits
Louisiana
2 (LAG38)
1 (LAG53)
12
LAG380000: Potable Water Treatment Plant
LAG530000: Waste Water Treatment Plant
Maine
0
12
No general permit.
Maryland
9
26
MDG670000: General Permit for Tanks and Pipes, and Other
Liquid Containment Structures at Facilities other than Oil
Terminals
Massachusetts
55 (MAG64)
8 (MAG07)
6
MAG640000: Water Treatment Facility Discharges
MAG070000: Construction Dewatering
Michigan
34
8
MIG640000: Wastewater Discharge from Potable Water Supply
Minnesota
38 (MNG64)
1 (MNG82)
b
22
MNG640000: Treated Filter Backwash Water from Water
Treatment Facilities
Mississippi
0
19
No general permit.
Missouri
142
(MOG640)
7 (MOG641)
1 (MOG25)
b
37
MOG640000 - Water Treatment Plant Filter Backwash
MOG641000 - Backwash Water from Water Softening Units
Montana
1
15
MTG770000: Disinfected Water Discharges
Nebraska
0
27
No general permit.
Nevada
0
3
No general permit.
New Hampshire
3
None listed in
PCS
NHG640000: Water Treatment Facility Discharges
New Jersey
0
33
No general permit.
New Mexico
0
7
No general permit.
New York
0
52
No general permit.
North Carolina
0
170
No general permit.
North Dakota
0
26
No general permit.
Ohio
0
142
No general permit.
Oklahoma
4
31
OKG38: Filter Backwash Discharges from Potable Water
Treatment Plants
Oregon
63 (ORG38)
1 (ORG75)
b
5
OR38 (OR-200-J on website): Discharge/Land Application of
Filter Backwash, Settling Basin, and Reservoir Cleaning Water
Pennsylvania
0
139
No general permit.
Puerto Rico
0
109
No general permit.
Rhode Island
0
5
No general permit.
South Carolina
58 (SCG64)
1 (SCG25)
11
SCG641000: Water Treatment Plant Discharges
With Maximum Total Residual Chlorine (TRC) Limits
SCG643000: Water Treatment Plant Discharges With Median
TRC Limits
SCG645000: Water Treatment Plant Discharges With the Lowest
TRC Limits
SCG250000: Utility Water Discharge
South Dakota
21
9
SDG07: Temporary Dewatering Activities
4-3

Drinking Water Industry Report Section 4 – Current State NPDES Permit Requirements
Table 4-1. Summary of Permit Information in the 2004 Permit Compliance System
State
WTPs in EPA’s Permit
Compliance System
a
General Permits for WTP Discharges
General
Permits
Individual
Permits
Tennessee
None listed
in PCS
111
TNG640000: Filter Backwash and Sedimentation Basin
Washwater from Water Treatment Plants
Texas
0
126
No general permit.
Utah
31
1
UTG640000: General Permit for Drinking Water Treatment
Plants
Vermont
0
2
No general permit.
Virginia
0
128
No general permit.
Virgin Islands
0
14
No general permit.
Washington
30
None listed in
PCS
WAG-64: Water Treatment Plant General Permit
West Virginia
86 (WVG64)
1 (WVG55)
b
24
WVG64 (WV0115754 on website): Water Treatment Plant
Wastewater Disposal Systems
Wisconsin
None listed
in PCS
None listed in
PCS
WI-0046540-4 (Process wastewater discharges): Potable Water
Treatment and Conditioning
Wyoming
1
12
WYG71: General Permit for Temporary Discharges
Total
971
1,855
Source: ERG, 2005.
a – Additional WTPs included in PCS, but omitted from table because of low flow or not applicable activities (by
state):
Arkansas: Eight additional plants have discharge coverage under general permit ARG550000 - Individual
Treatment Facilities with maximum design flow of ≤ 1,000 gallons/day.
Colorado: 12 additional plants have discharges covered under COG60 - Minimal Discharges (not specific to
drinking water treatment).
Massachusetts: One plant had discharges covered under a general permit for noncontact cooling water
(MAG25)
Minnesota: One plant had discharges covered under a general permit for noncontact cooling water (MNG25)
Missouri: One plant had discharges covered under a general permit for noncontact cooling water (MOG35)
North Carolina: Four additional plants discharge under general permit NCG510000, Groundwater Remediation
b – Permit not found on-line; discharge coverage unknown.
States commonly issue general permits for certain waste streams discharged by
WTPs. The most common waste stream covered by general permits is filter backwash water.
Residuals from solids settling (e.g., clarifiers, lagoons) are the second most common waste
stream covered. Residuals less commonly covered by general permits include water softening
discharges, membrane desalination concentrates, and ion exchange regeneration waste. Sections
6 and 7 of this document discuss the source water treatment operations and residuals generated in
more detail.
4-4

Drinking Water Industry Report Section 4 – Current State NPDES Permit Requirements
Table 4-2 lists the process wastewater discharges covered by drinking water
treatment industry-specific general permits identified in Table 4-1. In addition to the information
presented in the table, the Louisiana General Permit (LAG380000) also covers wastewater from
disinfection of source water, the South Carolina (SCG641000, SCG643000, and SCG645000)
and Tennessee (TNG640000) general permits also cover wash water from sedimentation basins.
Some general permits specifically prohibit discharges (or exclude them from permit coverage).
For example, the Oklahoma general permit (OKG38) requires no residual disinfectant in the
discharge (i.e., completely diluted in the backwash water during storage in detention ponds).
General permits may further limit applicability beyond type of WTP discharge.
Applicability requirements include providing general permit coverage for existing plants only
(MAG640000 and NHG640000) and limiting coverage to smaller dischargers using discharge
flow rate limits or production limits. General permits that limit coverage based on discharge
quantities, include the following:
• The Oklahoma general permit (OKG38) requires discharge of no more
than one million gallons per day;
• The California Central Coast general permit (CAG993001) requires
continuous maximum discharges to be specified in the permit, including
limiting desalination concentrate to 50,000 gallons per day; and
• The Washington general permit (WAG-64) requires a maximum
production capacity of 50,000 gallons per day (peak output based on 24-
hour production).
Other applicability requirements are also used to protect the receiving water. For
example, the Wisconsin general permit (WI-0046540-4) does not cover discharges containing
radium and arsenic (present in water supply). Additionally, a number of general permits do not
cover discharges to certain receiving streams (e.g., impaired waters); in those cases, WTPs need
to apply for an individual permit.
If the WTP does not meet the applicability of the general permit, an individual
permit must be obtained prior to discharge. The types of waste streams and pollutants covered by
4-5

Drinking Water Industry Report Section 4 – Current State NPDES Permit Requirements
an individual permit depend on the source water treatment operations, treatment chemicals, and
source water contaminants at a particular plant.
Table 4-2. Wastewater Discharges from WTPs Covered by General Permits
State
NPDES Permit
Number
Wastewater Discharges Covered by General Permit
Filter
Backwash
Solids
Removal
a
Water
Softening
b
Ion Exchange
Regeneration
Reverse
Osmosis
Concentrate
Wastewater
from Sludge
Dewatering
AR
ARG640000
X
X
CA
CAG9930001
X
c
CO
COG640000
X
X
CT
GP-002
X
X
X
X
X
IL
ILG64
X
X
X
KY
KYG640000
X
LA
LAG380000
X
X
X
MA
MAG640000
X
X
d
MD
MD670000
Overflow, flushing, disinfection, mechanical cleaning, or dewatering discharges
MI
MIG640000
X
X
e
MN
MNG640000
X
MO
MO-G640000
X
MO-G641000
X
f
X
f
MT
MTG770000
Disinfected water discharges
NH
NHG640000
X
X
d
OK
OKG38
X
OR
OR-200-J
X
SC
SCG641000,
SCG643000,
and SCG645000
X
X
SD
SD070000
X
g
TN
TN640000
X
UT
UTG640000
Not specified
WA
WAG-64
X
X
WV
WV0115754
X
h
X
h
WI
WI-0046540-4
X
i
X
i
X
i
WY
WYG710000
Not specified (temporary discharges)
Source: ERG, 2005.
a – Residuals from solids removal include sludge/blowdown from clarifiers, lagoons, etc. and filter sludge. The filter
sludge may be part of iron and/or manganese removal operations.
b – Water softening residuals may include ion exchange wash/rinse concentrates and sludge from sedimentation
basins or filters.
c – The California Central Coast Regional general permit covers discharges of desalination concentrate up to 50,000
gallons per day to ocean waters. This general permit does not cover discharges of desalination concentrate to inland
surface waters.
4-6

Drinking Water Industry Report Section 4 – Current State NPDES Permit Requirements
d – The Massachusetts and New Hampshire general permits cover the discharge of treated presedimentation
underflow and treated underflow from coagulation/settling processes using aluminum compounds or polymers as
coagulants.
e – The Michigan general permit No. 649000 (expires April 1, 2005) includes water softening discharges, except
from batch regenerated potassium permanganate iron removal and sodium zeolite softening. The general permit
effective April 1, 2005 does not cover any discharges from water softening.
f – The Missouri general permit (G641000) covers discharges of backwash water from water softening.
g – South Dakota general permit covers discharges from temporary dewatering activities. The discharges “must be
relatively uncontaminated and must not contribute nonconventional or toxic pollutant loadings to the receiving
stream.”
h – The West Virginia general permit covers treatment wastewater discharges and describes minimum treatment
requirements for sediment removal and total residual chlorine removal.
i – Discharges covered by the Wisconsin general permit (WI-0046540-4) include those from iron removal filters
(excluding batch regeneration by potassium permanganate (KMnO4) to surface water), demineralizers (excluding
sodium or potassium cycle ion exchange softeners), lime softeners, alum coagulation units, granular media filters,
reverse osmosis units, and other systems with similar discharges.
4.2 SUMMARY OF CURRENT POLLUTANT LIMITATIONS AND
REQUIREMENTS FOR WATER TREATMENT PLANTS: GENERAL
AND INDIVIDUAL PERMITS
The most common pollutants regulated in general permits include aluminum, iron,
manganese, pH, settleable solids, total residual chlorine (TRC), and total suspended solids (TSS).
In addition, NPDES permits for membrane desalination and ion exchange plants may also
require limits or monitoring of chlorides and total dissolved solids (TDS) (ERG, 2005).
WTPs not covered under a general discharge permit must apply for an individual
NPDES permit. EPA reviewed individual permits from the following states:
• Alabama;
• Alaska;
• Arizona;
• California;
• Florida;
• Illinois;
• Indiana;
• Iowa;
• Kansas;
• Massachusetts;
• Missouri;
• Montana;
• Nebraska;
• Nevada;
• North Carolina;
• Ohio;
• Pennsylvania;
• Puerto Rico;
• Texas;
• Washington, DC; and
• Wisconsin.
The common pollutants regulated in individual permits include aluminum, copper, dissolved
oxygen, iron, lead, pH, temperature, TRC, TSS, and turbidity. Other pollutants that may be
included in WTP permits based on source water characteristics or treatment chemicals used
4-7

Drinking Water Industry Report Section 4 – Current State NPDES Permit Requirements
include ammonia, arsenic, biochemical oxygen demand (BOD), cadmium, manganese, oil and
grease, settleable solids, total phosphorus, and zinc. In addition, NPDES permits for membrane
desalination and ion exchange plants may also require limits or monitoring of chlorides and
TDS. Table 4-3 lists the range of pollutant limitations in general and individual NPDES permits
reviewed by EPA as part of the industry review (ERG, 2005 and ERG, 2008).
Table 4-3. Range of Pollutant Limitations From a Sample of General and Individual
NPDES Permits
Pollutant
General NPDES Permits
Individual NPDES Permits
States with
Limitations or
Reporting
Requirements
Monthly
Average
Limitation
a
Daily
Maximum
Limitation
a
States with
Limitations or
Reporting
Requirements
Monthly
Average
Limitation
b
Daily
Maximum
Limitation
b
Aluminum
Majority of
state general
permits
0.75 to 1
mg/L
1.5 to 10
mg/L
California
Missouri
Montana
Pennsylvania
Washington, DC
1 to 4 mg/L
1.5 to 8 mg/L
Ammonia
Colorado
Report only
California
Puerto Rico
Report only
1 mg/L
Arsenic
Michigan
0.150 mg/L
0.680 mg/L
Alaska
Arizona
California
Puerto Rico
0.036 mg/L
0.00018 to
0.080 mg/L
Biochemical
oxygen
demand
(BOD)
None
—
—
California
Florida
Illinois
Puerto Rico
10 to 20 mg/L
5 to 30 mg/L
Cadmium
None
—
—
California
Florida
Missouri
0.002 to
0.0093 mg/L
0.004 to
0.042 mg/L
Chlorides
Illinois
Louisiana
Missouri
—
250 to 1,000
mg/L
California
Florida
—
150 mg/L
Copper
Connecticut
Wisconsin
—
<1.09 mg/L
Arizona
California
Florida
Massachusetts
Puerto Rico
0.0031 to
0.007 mg/L
0.0029 to
0.500 mg/L
Dissolved
oxygen
None
—
—
Alaska
California
Florida
Puerto Rico
Minimum: 2.0 to 7.0 mg/L
4-8

Drinking Water Industry Report Section 4 – Current State NPDES Permit Requirements
Table 4-3. Range of Pollutant Limitations From a Sample of General and Individual
NPDES Permits
Pollutant
General NPDES Permits
Individual NPDES Permits
States with
Limitations or
Reporting
Requirements
Monthly
Average
Limitation
a
Daily
Maximum
Limitation
a
States with
Limitations or
Reporting
Requirements
Monthly
Average
Limitation
b
Daily
Maximum
Limitation
b
Iron
Majority of
state general
permits
1 to 5 mg/L
2 to 10 mg/L
Alaska
Florida
Illinois
Indiana
North Carolina
Pennsylvania
Washington, DC
1.8 to 2 mg/L
0.3 to 4.1
mg/L
Lead
Wisconsin
—
—
Arizona
California
Missouri
Puerto Rico
0.003 to
0.0081 mg/L
0.0044 to
0.210 mg/L
Manganese
Majority of
state general
permits
0.0043 to 1
mg/L
0.019 to 3
mg/L
Arizona
Pennsylvania
Puerto Rico
1 mg/L
0.05 to 2
mg/L
Oil and
grease
California
Colorado
25 mg/L
10 to 75 mg/L
California
Massachusetts
Puerto Rico
10 mg/L
10 to 15 mg/L
pH
Majority of
state general
permits
6.0 to 9.0 s.u.
Majority of states
reviewed
6.0 to 11.0 s.u.
Phosphorus
Michigan
1 mg/L
—
California
Florida
Missouri
Puerto Rico
1 mg/L
1 mg/L
Settleable
solids
California
Missouri
Oregon
Tennessee
Washington
0.1 to 2.0
mL/L
0.1 to 3.0
mL/L
California
Missouri
North Carolina
0.1 mL/L
0.2 to 0.3
mL/L
Temperature
None
—
—
California
Massachusetts
Nevada
Puerto Rico
—
86 to 100°F
±5°F: effect
on receiving
stream
Total
dissolved
solids (TDS)
Colorado
Connecticut
Illinois
—
1,000 to
1,500 mg/L
Alaska
California
Illinois
Nevada
80 to 800
mg/L
95 to 1,500
mg/L
Total residual
chlorine
(TRC)
Majority of
state general
permits
0.03 to 1
mg/L
<0.02 to 1
mg/L
c
Majority of states
reviewed
0.01 to 0.29
mg/L
0.002 to 1.3
mg/L
Total
suspended
solids (TSS)
Majority of
state general
permits
15 to 30 mg/L
20 to 60 mg/L
Majority of states
reviewed
15 to 70 mg/L
5 to 150 mg/L
4-9

Drinking Water Industry Report Section 4 – Current State NPDES Permit Requirements
Table 4-3. Range of Pollutant Limitations From a Sample of General and Individual
NPDES Permits
Pollutant
General NPDES Permits
Individual NPDES Permits
States with
Limitations or
Reporting
Requirements
Monthly
Average
Limitation
a
Daily
Maximum
Limitation
a
States with
Limitations or
Reporting
Requirements
Monthly
Average
Limitation
b
Daily
Maximum
Limitation
b
Turbidity
California
75 NTU
225 NTU
California
Massachusetts
Nevada
Puerto Rico
6 to 50 NTU
5 to 150 NTU
Zinc
Connecticut
Wisconsin
—
<2.0 mg/L
California
Missouri
Puerto Rico
0.061 to
0.093 mg/L
0.09 to 50
mg/L
Sources: ERG, 2005; ERG, 2008.
NTU—Nephelometric turbidity units
a – Limitations may be less than range presented for certain receiving streams (e.g., small streams, impaired waters).
b – Some states may only require monitoring and reporting (i.e., no numerical limitations).
c – One general permit allows up to 3.0 mg/L TRC discharge to ground water.
One of the trends in the drinking water treatment industry is the increased use of
membrane desalination operations. Between 1992 and 1999, the number of desalination plants in
the United States with production of 25,000 gallons per day or more increased from 103 to 203
plants (Mickley, 2001). Residuals from desalination include concentrates. Due to large volumes
and high TDS concentrations, WTPs have difficulty disposing of concentrates unless discharge
to surface water is an option. Most membrane desalination plants do not treat the concentrate
prior to discharge. Other waste management options include indirect discharge, land application,
landfill disposal, and underground injection (Malmrose, et al., 2004). These other waste
management options often include certain regulations that must be met by the WTP.
Typical permit limitations for direct discharge of desalination concentrate include
TDS, TSS, salinity, and contaminants specific to the source water such as nutrients (nitrogen and
phosphorus), arsenic, barium, and radionuclides. If the discharge is potentially highly saline,
WTPs may dilute the discharge with source water, wastewater treatment plant effluent, or
cooling water. Also, concentrates for membrane systems treating ground water may contain low
dissolved oxygen levels that can adversely impact the receiving stream (Malmrose, et al., 2004).
4-10

Drinking Water Industry Report Section 4 – Current State NPDES Permit Requirements
4.3 REFERENCES
Eastern Research Group (ERG), 2005. Memorandum, Summary of State
Requirements/Prohibitions for DWT Facilities, March 15, 2005. DCN DW00149.
ERG, 2008. MS Excel Spreadsheet: Permit Summary Individual, May 2008. DCN DW03733.
Malmrose, et al., 2004. Paul Malmrose, Jim Lozier, Michael Mickley, Robert Reiss, Jerry
Russell, James Schaefer, Sandeep Sethi, Jennifer Manuszak, Robert Bergman, and Khalil Z.
Atasi, Residual Management Research Committee Subcommittee on Membrane Residual
Management, “2004 Committee Report: Residuals Management for Desalting Membranes,”
Jour. AWWA, 96:12:73. American Water Works Association (AWWA), December 2004.
Document Control Number (DCN) DW00032.
Mickley, 2001. Michael C. Mickley. Membrane Concentrate Disposal: Practices and
Regulations. Prepared for U.S. Department of the Interior by Mickley and Associates. Boulder,
Co. September. DCN DW00028.
4-11

SECTION 5
SOURCE WATER QUALITY
Drinking water sources include ground water and surface water. Ground water
comes from wells drilled into underground aquifers (geologic formations containing water).
Surface water is obtained from rivers, lakes, and reservoirs open to the atmosphere.
Source water may contain undesirable contaminants that must be removed from
the drinking water. These contaminants enter the water supply via natural sources or from human
activities. Table 5-1 presents common source water contaminants and their environmental,
agricultural, and industrial sources. Source water quality can also vary based on geographic
region. This section discusses factors that influence source water quality (Section 5.1), compares
ground water and surface water quality (Section 5.2), and discusses how the Safe Drinking Water
Act (SDWA) addresses source water protection (Section 5.3).
Table 5-1. Common Source Water Contaminants and Sources
Contaminant
Sources
Naturally Occurring
Microorganisms
Wildlife and soils; microorganism-containing wastes in runoff from nonpoint
sources, including animal wastes; and other point source discharges which are
not disinfected
Radionuclides: All except beta
particles and photon emitters
Erosion of natural deposits
Metals (e.g., arsenic, cadmium,
chromium, lead, and selenium)
Erosion of natural deposits
Nitrates and nitrites
Erosion of natural deposits
Fluoride
Erosion of natural deposits
From Human Activities
Microorganisms
Human and animal wastes
Radionuclides: Beta particles
and photon emitters
Decay of man-made deposits
Metals
Mining; construction; industrial discharges; runoff from orchards, croplands, and
landfills; lead and copper from household plumbing materials
Nitrates and nitrites
Runoff from fertilizer use; leaching of septic tanks or sewage
Organics
Runoff from herbicide and pesticide use, industrial discharges; emissions from
incineration or combustion; household wastes such as cleaning solvents
Source: U.S. EPA, 2008.
5-1

Drinking Water Industry Report Section 5 – Source Water Quality
5.1 FACTORS THAT INFLUENCE SOURCE WATER QUALITY
The factors that influence the quality of source water—both ground water and
surface water—include naturally-occurring attributes (climate, geology, soil type, land cover,
hydrology, precipitation and runoff, and wildlife) and man-made attributes (land management
practices and runoff or discharge from point and nonpoint sources). The Safe Drinking Water
Act (SDWA) Amendments of 1996 required states to develop and implement source water
assessment programs (SWAPs) to analyze existing and potential threats to the quality of the
public drinking water throughout the state. Using these programs, most states have completed
source water assessments for every public water system - from major metropolitan areas to the
smallest towns (http://cfpub.epa.gov/safewater/sourcewater/sourcewater.cfm?action=Programs#swap).
Using baseline water quality data, water treatment plants (WTPs) are designed with the treatment
technologies necessary to produce potable water (U.S. EPA, 1999a). Source water quality
impacts the design of the WTP, the treatment chemicals used, and the quantity and composition
of the residuals generated.
Source water quality may vary over time or in seasonal cycles. Land uses, such as
agriculture, urban development, and industrial sites, and the watershed management (i.e., the
management of the land around a waterway) are the variables that most affect the source water
quality conditions over time. For example, agricultural practices that affect source water quality
include irrigation, field drainage, and chemical and biosolids application to crops and soil.
Industrialization and urbanization within the watershed may affect source water quality due to
changes in storm water runoff. As the land management practices change, WTPs adjust their
operations and treatment chemical usage to meet drinking water quality standards. Changes in
the source water quality (e.g., additional solids due to increased soil runoff; increased nutrient
content in the source water due to fertilizer use) also affect the generation and composition of
residuals. These land management practices are responsible for additional treatment over the
baseline conditions of the source water.
Watershed management includes strategies and plans to assess and maintain a
water resource within a specified drainage area. The overall strategy is to maintain or improve
the quality of water (drinking, recreational, or industrial) that is derived from the watershed and
5-2

Drinking Water Industry Report Section 5 – Source Water Quality
to comply with the various statutes like the Clean Water Act (CWA), the SDWA, and state/local
requirements.
The industrialization and urbanization of rural land increases the amount of runoff
into source water (U.S. EPA, 2001). The increased runoff of silt and sediment increases the
amount of solids that WTPs must remove from source water, and, ultimately, the amount of
residual solids. To remove these additional solids from drinking water, WTPs may need to spend
additional money on operations, treatment chemical usage, residuals treatment operations, and
residuals disposal costs. One way to reduce these costs is to have a strong cooperative watershed
management program that maintains the quality of the source water.
Some of the less obvious runoff effects are caused by landscaping chemicals from
lawns and gardens, as well as oil and hydrocarbons from roadways. The increased impervious
surfaces of urban and industrial areas do not retain runoff, and the quality and quantity of both
surface and ground water are adversely impacted. Increased runoff can lead to other watershed
related problems such as flow modifications, erosion, introduction of chemical and
microbiological pollutants, accumulation of sediments, habitat loss, ecosystem disruption, and
the possible introduction of invasive species. The additional pollutants present in the source
water must be removed by WTPs to meet drinking water standards and customer demands.
Watershed protection can be a key pollution prevention option to reduce residuals
from source water treatment. For example, New York City is investing $1.2 billion to safeguard
its upstate reservoir system in hopes of reducing or eliminating the estimated $6 to 8 billion
required for a filtration plant to treat an unprotected watershed. Also, New Jersey has a multiyear
master plan for long-term funding and acquisition of watershed properties to protect source water
quality (Ernst, 2004).
5.2 COMPARISON OF GROUND WATER AND SURFACE WATER
QUALITY
Most ground water is naturally filtered as it passes through layers of the earth into
underground reservoirs known as aquifers. Ground water generally contains less organic material
than surface water and may not need to undergo as many treatment steps. Surface water collects
5-3

Drinking Water Industry Report Section 5 – Source Water Quality
a wide variety of contaminants from watershed drainage, agricultural practices, and urban
sources. Thus, surface water has more variable and extensive treatment requirements.
EPA’s Office of Ground Water and Drinking Water (OGWDW) completed a
review of contaminant occurrences in the source water for drinking water systems (U.S. EPA,
1999b). The purpose of the review was to enhance the scientific understanding of the occurrence
of chemical contaminants in public drinking water systems and to refine the basis for the
monitoring of these contaminants. The review found the following occurrence results for ground
water and surface water:
• Volatile organic compounds (VOCs) are more common in surface water;
however, exceedances of the EPA MCLs are nearly equal for surface and
ground water systems.
• Some VOCs are not geographically centralized (i.e., they are present in
source water in all states
17
studied). These VOCs include ethylbenzene,
cis-1,2-dichloroethane, tetrachloroethylene (PCE), trichloroethylene
(TCE), vinyl chloride, 1,1,1-trichlorethane, and xylenes.
• Inorganic chemicals are common in both surface and ground water, but
ground water concentrations tend to be higher.
• Synthetic organic chemicals (SOC) are more common in surface water.
Section 8 of this report discusses pollutants of concern for surface water and
ground water.
5.3 SOURCE WATER PROTECTION UNDER THE SDWA
In addition to establishing drinking water requirements, the 1996 Amendments to
the SDWA outlined measures to ensure the quality of drinking water by protecting the source
water. The measures include source water assessments, providing information to the public
(consumer confidence reports), and providing federal funds for source water assessments and
protection.
17
The study included source water for drinking water systems in the following states: Alabama, California, Illinois,
Indiana, Iowa, Massachusetts, Michigan, Montana, New Jersey, New Mexico, Ohio, and Oregon.
5-4

Drinking Water Industry Report Section 5 – Source Water Quality
To give water systems and community members the information needed to decide
how to protect their drinking water sources, the SDWA requires states to develop EPA-approved
programs to carry out assessments of all source waters in the state. The source water assessment
is a study that defines the land area contributing water to each public water system, identifies the
major potential sources of contamination that could affect the drinking water supply, and then
determines how susceptible the public water supply is to this potential contamination. Water
systems and communities can then use the publicly-available study results to reduce potential
sources of contamination and protect the source water.
Community water systems are also required to provide consumer confidence
reports, or annual water quality reports, to the public each year. The report explains where the
supplied drinking water comes from and what contaminants might be in the drinking water. The
consumer confidence reports summarize information regarding sources used (e.g., rivers, lakes,
reservoirs, or aquifers), any detected contaminants, compliance, and educational information.
EPA provides funding to states through the Drinking Water State Revolving Fund
(DWSRF) for source water assessment and protection activities. Source water protection
approaches are tailored to each unique local situation. Although most source water protection
efforts are primarily led by the system (or utility), state, or locality, a variety of federal tools can
be used, such as those available through the CWA, Underground Injection Control Program, and
various agricultural programs. In addition, a number of national nongovernmental organizations,
such as the American Water Works Association (AWWA), the National Rural Water Association
(NRWA), the National Association of Counties (NACo), and the Trust for Public Lands (TPL),
are active in the realm of source water protection. One of EPA's roles is to encourage
partnerships and provide information to those directly involved in source water protection.
5.4 REFERENCES
Ernst, Caryn, 2004. Protecting the Source: Land Conservation and the Future of America’s
Drinking Water. Published by the Trust for Public Land and AWWA. DCN DW03759.
5-5

Drinking Water Industry Report Section 5 – Source Water Quality
U.S. EPA, 1999a. Guidance Manual for Conducting Sanitary Surveys of Public Water Systems;
Surface Water and Ground Water Under the Direct Influence (GWUDI) (EPA 815-R-99-016),
Office of Water, Washington, DC. DCN DW01147.
U.S. EPA, 1999b. A Review of Contaminant Occurrence in Public Water Systems (EPA 816-R-
99-006), Office of Water, Washington, DC. DCN DW00941.
U.S. EPA, 2001. Protecting and Restoring America’s Watersheds: Status, Trends, and Initiatives
in Watershed Management (EPA 840-R-00-001), Office of Water, Washington, DC. DCN
DW00934.
U.S. EPA, 2008. National Primary Drinking Water Standards (List of Drinking Water
Contaminants and MCLs) (original document published in 2003, DCN DW00657), Office of
Ground Water and Drinking Water, Washington, DC. Retrieved from
http://www.epa.gov/safewater/contaminants/index.html Accessed April 2008 for updates.
5-6

SECTION 6
SOURCE WATER TREATMENT TECHNOLOGIES
Treatment of source water removes contaminants that are unhealthy or
undesirable for consumption. The type of treatment operation performed at a drinking water
treatment plant (WTP) and treatment chemicals used depend on the contaminants present in the
source water. The removed contaminants and treatment chemical composition impact the content
and quantity of residuals generated. This section discusses the source water treatment operations
and treatment chemicals used that impact the content and quantity of residuals generated.
WTPs strive to add sufficient treatment chemicals to source water to remove
contaminants without adding excessive levels of additional pollutants (i.e., treatment chemical
active ingredients and impurities). AWWA began assembling consensus standards for different
aspects of drinking water production about 100 years ago and updates them periodically.
Included in those consensus standards are best engineering judgments for the different chemicals
added to drinking water.
About 20 years ago, the National Sanitation Foundation (NSF) and a consortium
of stakeholders established minimum human health effects requirements for any chemicals added
directly to drinking water. The American National Standards Institute (ANSI)/NSF Standard 60
recommends, when available, that EPA MCLs (U.S. EPA, 2008b) be used to determine the
acceptable level for a treatment chemical in the finished drinking water. If an MCL is not
available, ANSI/NSF Standard 60 provides criteria to conduct a toxicological risk assessment for
the chemical.
There are many different approaches to removing source water contaminants. In
addition to the characteristics of the source water, the size of the system or plant may be a factor
when selecting or implementing new source water treatment operations. For example, larger
systems have in general a larger number of technology options to select from and can take
advantage of economies of scale that can reduce both capital and operational expenses, allowing
for a lower per unit of treated water cost. The larger systems can also spread the costs incurred to
6-1

Drinking Water Industry Report Section 6 – Source Water Treatment Technologies
install and operate source water treatment over a larger customer base. Common treatment
operations for all system sizes that affect residuals content and quantity generated are discussed
in this section. The source water treatment operations discussed include the following:
• Conventional filtration, direct filtration, and filtration only (Section 6.1);
• Precipitative softening (Section 6.2);
• Membrane separation (Section 6.3);
• Ion exchange (Section 6.4);
• Activated carbon (Section 6.5);
• Disinfection (Section 6.6); and
• Other chemical additions (Section 6.7).
6.1 CONVENTIONAL FILTRATION, DIRECT FILTRATION, AND
FILTRATION ONLY
Conventional filtration is the most common treatment train at WTPs and is the
primary treatment used at 63 percent of WTPs. It is a series of processes including coagulation,
flocculation, sedimentation, and filtration that result in substantial particulate removal from the
source water. Figure 6-1 shows a typical conventional filtration treatment plant flow diagram.
Direct filtration is another treatment train operated at WTPs, where plants perform
coagulation, flocculation, and filtration without sedimentation. Unlike conventional filtration, the
floc is removed at the filter rather than at the sedimentation basin (National Drinking Water
Clearinghouse, 1996b). Some treatment plants perform filtration without coagulation or
flocculation, referred to as filtration only.
The types of processes used at the WTP depend on the characteristics of the
source water. Source water with high solids content may require pretreatment, or
presedimentation. The following subsections focus on the individual processes that WTPs use to
remove particulates (or solids) from the source water either as stand-alone processes or in series.
6-2

Drinking Water Industry Report Section 6 – Source Water Treatment Technologies
Coagulation
(rapid mix
process)
Flocculation
(separates
suspended solids
from water by
creating “floc”)
Sedimentation
(heavy particles
settle to bottom)
Filtration
(removes finer
particles)
Finished Water to
Storage and
Distribution
Settled Solids
(continuously or
periodically
removed)
Coagulant
Raw Water
Disinfectant
Clarified
Water
Filter Backwash (recycled)
Figure 6-1. Typical Conventional Filtration Treatment Plant Flow Diagram (U.S. EPA, 2002a)
6-3

Drinking Water Industry Report Section 6 – Source Water Treatment Technologies
Residuals generated by solid removal processes include filter backwash water,
filter-to-waste, and coagulation sludge (i.e., underflow streams removed from sedimentation or
settling tanks). The residuals contain solids from the source water and chemicals added by the
WTP to aid in solids removal. In addition, filter backwash and filter-to-waste streams may
contain residual disinfectants.
6.1.1 Presedimentation
Presedimentation is a pretreatment process operated at the head of the WTP (e.g.,
in a sedimentation basin) or prior to intake (e.g., within a reservoir). Its primary purpose is to
remove a significant amount of readily settleable and suspended solids and other contaminants in
the source water prior to other water treatment operations (e.g., coagulation and filtration). WTPs
might add treatment chemicals during presedimentation; however, the primary removal
mechanism is gravity settling. The process removes relatively high concentrations of easily
settled solids (e.g., sand and silt). By allowing adequate detention time in the basin, coarser and
other easily settleable particles drop out of the source water. To aid settling, WTPs may add
polymers and other coagulants. The settled solids are removed continuously (or in frequent
batches) via an underflow pipe.
6.1.2 Coagulation, Flocculation, and Sedimentation
Coagulation, flocculation, and sedimentation are water treatment processes
performed in mixing tanks and sedimentation basins. WTPs operate one or more of the processes
to remove as much source water solid matter as possible. Most plants follow coagulation,
flocculation, and sedimentation with filtration to remove finer solid particles such as suspended
solids, colloids, and color (indicative of dissolved organic material).
At the clarification basins, coagulants and flocculants are added to the source
water. Agitation of the water causes collisions between suspended particles, forming
agglomerated solids. The solids settle to the bottom of the basin and are removed via an
underflow pipe. An additional sedimentation basin may be used to allow further solids settling.
6-4

Drinking Water Industry Report Section 6 – Source Water Treatment Technologies
Coagulants and flocculants added to the raw water include metal salts (e.g.,
aluminum sulfate and ferrous sulfate) and polyelectrolytes. To optimize solids removal, plants
may adjust the pH. Coagulant chemicals carry multivalent positive charges, which, when
dissolved in water, tend to neutralize the negative charges on the surface of the particulate
matter. This allows the small particles to approach each other, overcome electrostatic repulsion,
and combine. As the particles grow larger, they become heavier and gravity aids their settling to
the bottom of the tank.
Inorganic coagulants (e.g., aluminum sulfate, aluminum chloride, ferrous sulfate,
and ferric chloride) are used by many WTPs. The trivalent forms of aluminum and iron (Al
+++
,
Fe
+++
) are insoluble at normal drinking water treatment operating conditions so very little metal
is carried into the finished product (Tchobanoglous, et al., 2003). In addition to inorganic
chemicals, a large variety of organic-based polymers are employed as coagulant aids either
independently or in concert with the inorganic coagulation aids. About 1,100 different
formulations of polymer beads, polyacrylamide, polyamines, and polydimethylammonium
chloride are listed in ANSI/NSF Standard 60 and used to promote the removal of turbidity from
drinking water. Some of these chemicals are also referred to as filtration aids, but function the
same way as coagulants.
Residuals are generated as underflow discharges from the sedimentation tanks.
These residuals contain source water contaminants, as well as chemicals added to aid solid
removal and formulation impurities in the added treatment chemicals.
6.1.3 Filtration
After solids settling, the source water passes through filters to remove finer
particles and metals. Various types of filter media may be used by WTPs, including permeable
fabric and porous beds. The types of filters used by WTPs include the following:
• Slow sand: consists of a bed of fine sand above a gravel layer and
underdrain system. This type of filter is used for low-flow rates and might
be performed without other solids removal treatment steps (i.e., filtration
6-5

Drinking Water Industry Report Section 6 – Source Water Treatment Technologies
only). Slow sand filters are not suitable for high turbidity source waters.
(National Drinking Water Clearinghouse, 1996b)
• Rapid sand: consists of a bed of sand above several layers of gravel in
varying sizes.
• Pressure: similar to rapid sand filters but the operation is housed within a
cylindrical tank and the water passes through the filter while under
pressure generated by a pump rather than by gravity.
• Diatomaceous earth: consists of a layer of diatomaceous earth above a
septum or filter element. Most suitable for low turbidity and low bacterial
count source water. Coagulants and filter aids are required for effective
virus removal. (National Drinking Water Clearinghouse, 1996b)
• Multimedia: consists of layers of various sizes of gravel, high-density
garnet, sand, and anthracite coal.
• Membrane filters: include ultrafilters and microfilters. These membranes
use pressure as the driving force and are designed to remove particulates
smaller than 10 micrometers (discussed in Section 6.3).
The filtration process removes suspended solids by mechanical straining—
trapping them between grains of the filter medium (e.g., bed of sand). Filtration also uses
adhesion to remove solids; suspended solids stick (or adhere) to the surface of the filter material
or previously deposited solids. In addition to mechanical removal, slow sand filters trap
microorganisms that break down algae, bacteria, and other organic matter. (National Drinking
Water Clearinghouse, 1996b)
Slow sand and diatomaceous earth filtration are older filtration techniques that are
effective in removing suspended particles and some microbes. Many older systems abandoned
the use of these filter media due to slower filtration rates and the larger required size of the slow
sand and diatomaceous earth filter beds (about 10 times that of the newer systems). Low
filtration rate and large filter size both translate into higher operating costs. As a result, WTPs
have switched to other fine particle removal systems like membranes or multimedia filters.
WTPs may operate filtration systems without coagulation; these plants are
typically smaller (less than 50,000 people served) and treat ground water (U.S. EPA, 2008a).
6-6

Drinking Water Industry Report Section 6 – Source Water Treatment Technologies
Smaller WTPs may also use adsorption (e.g., activated carbon), rather than filtration to remove
certain contaminants (see Section 6.5).
Residuals from filtration operations include filter backwash (finished drinking
water flushes out solids and contaminants trapped in the filter) and filter-to-waste (initial
permeate after the filter has been brought on line). The residuals may contain source water
contaminants (e.g., solids), treatment chemical active ingredients and impurities, and residual
disinfectant added prior to filtration or in the finished water used to backwash the filter.
6.2 PRECIPITATIVE (LIME) SOFTENING
Drinking water that contains elevated levels of divalent cations, mostly calcium
and magnesium, can produce customer complaints that revolve around appliance malfunctions
(pipe scaling) and aesthetic concerns (water spots). These compounds present in the source water
contribute to the water’s “hardness.” Plants remove these compounds from the water by
precipitative, or chemical, softening.
Precipitative softening is the removal of divalent cations by increasing the pH and
altering the bicarbonate equilibrium. As the pH increases to about 9.5, the increased alkalinity
extracts a hydrogen atom from the bicarbonate and forces the equilibrium toward the carbonate
species, resulting in a precipitate of insoluble calcium carbonate. The chemical reaction is shown
in Equation 6-1 (Manahan, 1993).
Ca
2+
+ 2HCO
3
-
+ Ca(OH)
2
→ 2CaCO
3
(solid) + 2H
2
O (Eq. 6-1)
If the pH is increased to about 11, magnesium is precipitated as a hydroxide, as shown in
Equation 6-2.
Mg
2+
+ 2OH
-
→ Mg(OH)
2
(solid) (Eq. 6-2)
The softening process increases pH and leaves excess calcium hydroxide in the
water. After softening, pH is reduced by the conversion of excess calcium hydroxide to solid
calcium carbonate using carbon dioxide, as shown in Equation 6-3.
6-7

Drinking Water Industry Report Section 6 – Source Water Treatment Technologies
CO
2-
+ Ca(OH)
2
→ CaCO
3
(solid) + H
2
O (Eq. 6-3)
Calcium oxide (lime) is usually the chemical of choice to affect the pH changes
necessary for precipitative softening, but in some cases, sodium carbonate (soda ash) is used.
Plants add lime to remove carbonate hardness―bicarbonates of calcium and magnesium, or
plants add lime and soda ash to remove carbonate hardness and non-carbonate
hardness―sulfates, chlorides, or nitrates of calcium and magnesium. The precipitative softening
process is usually integrated with other treatment processes, particularly conventional or direct
filtration (see Section 6.1). The precipitated solids are removed from the bottom of sedimentation
or settling tanks (underflow), generating a residual waste stream—referred to as softening
sludge.
Due to cost and operating concerns, not all of the hardness is removed. By a
combination of pH control, treatment bypass, and blending, plants customize the precipitative
softening operation to the initial water quality conditions and the customer demands. The
hardness level in the drinking water typically ranges between 80 and 100 mg/L (ASCE/AWWA,
1997).
6.3 MEMBRANE SEPARATION
Membranes are used to separate components of a liquid stream into useable and
waste products. Membrane systems are characterized by the driving force needed to effect
separation (e.g., pressure-driven or electrical-driven separation). Membrane separation
techniques used to treat source water include the following:
• High-pressure technologies, such as reverse osmosis (RO) and
nanofiltration (NF);
• Low-pressure technologies, such as microfiltration (MF) and ultrafiltration
(UF); and
• Electrical-driven technologies, such as electrodialysis (ED) and
electrodialysis reversal (EDR).
6-8

Drinking Water Industry Report Section 6 – Source Water Treatment Technologies
WTPs using membrane separation are typically smaller plants (serving less than
50,000 people). From EPA’s 2007 industry questionnaire, all plants that operated membranes
served less than 150,000 people (U.S. EPA, 2008a).
WTPs may use membrane operations to remove salt from saline or brackish
water. Brackish water typically contains between one and 35 parts per thousand (ppt) salt.
Seawater typically contains approximately 35 ppt of salt
18
(USGS, 2007). The removal of salt
from source water is called desalination. Desalination processes include RO, NF, ED, and EDR.
Residuals from MF and UF include filter backwash and spent cleaning solutions.
Residuals from membrane desalination include the concentrate or “reject” stream and spent
cleaning solutions. The following subsections discuss the desalination processes and MF and UF
processes in more detail.
6.3.1 Reverse Osmosis and Nanofiltration
Most membranes use pressure as the driving separation force. Generally the
smaller the pore size in the membrane, the higher the driving force (pressure) required to
accomplish separation. For source water treatment, the application determines the driving force
and thus the type of membrane. If the treatment application is removal of dissolved contaminants
(hardness, salinity, arsenic, radioactive cations), then WTPs use high pressure systems like NF or
RO. These systems also can remove dissolved organic material, biological contaminants, and
suspended solids.
Pretreatment is typically used to remove biological material and particulates. The
use of NF or RO requires clean source water, with a significant amount of pretreatment to
remove the majority of suspended solids so that the membrane will not quickly clog. The high
pressure and additional pretreatment needed to operate RO and NF systems can translate into
high operating and maintenance costs.
18
Fresh water contains less than one ppt (or 1,000 parts per million) salt (USGS, 2007).
6-9

Drinking Water Industry Report Section 6 – Source Water Treatment Technologies
NF and RO use semipermeable membranes to remove contaminants from the
source water. These systems operate at pressures between 75 pounds per square inch gauge
(psig) and 1,200 psig (Malmrose, et al., 2003). Figure 6-2 presents a cross-section of a reverse
osmosis membrane.
Permeate
Permeate
Pressurized Feed
Concentrate
Membrane
Membrane
Figure 6-2. Reverse Osmosis Cross-Flow Membrane (The Merit Partnership, 2002)
6.3.2 Microfiltration and Ultrafiltration
If the treatment application is particulate and suspended solids removal, lower
pressure membrane systems may be used. MF and UF systems can effectively remove turbidity,
metals such as iron, manganese and arsenic, as well as protozoan like Cryptosporidium.
Dissolved organics may be removed when assisted by an adsorption agent (e.g., powdered
activated carbon). UF systems can also remove viruses from the source water without additional
treatment (Malmrose, et al., 2003).
Pretreatment of source water is desirable but if the source is relatively clean,
pretreatment may not be necessary. Applications of MF and UF systems are becoming more
common as replacement for small, older conventional treatment systems. Lower capital costs,
lower operating costs, and improved performance are reasons for their increased use.
6-10

Drinking Water Industry Report Section 6 – Source Water Treatment Technologies
MF and UF systems use porous, hollow-fiber membranes to remove source water
contaminants. The membrane pore size for MF systems typically ranges between 0.1 and 10
micrometers (μm). UF pore size is smaller, ranging from 0.01 to 0.4 μm. These systems typically
operate at pressures less than 40 psig (Malmrose, et al., 2003).
6.3.3 Electrodialysis and Electrodialysis Reversal
Dissolved contaminants in the source water may be removed using electrodialysis
membranes, which are ion exchange membranes that use electrical current to separate the
contaminants from the water (Malmrose, et al., 2004). This operation is primarily used to desalt
brackish water. Electrodialysis is not effective in removing non-charged solutes, such as silica,
pathogens, and dissolved organics.
Electrodialysis uses alternating pairs of cation (positively charged) and anion
(negatively charged) membranes positioned between two oppositely charged electrodes.
Channeled spacers between the membranes create parallel flow streams across the membrane
surface. The source water is pumped into the flow channels. When voltage is applied, the
electrical current causes ions from the source water to migrate toward the oppositely charged
electrodes, where the ions become restrained in the polarized membranes. Cations are attracted
to the negatively charged electrodes, pass through the positively charged membranes, and
become restrained by the negatively charged membrane. Anions are attracted to the positively
charged electrodes, pass through the negatively charged membranes, and become restrained by
the positively charged membranes. (Malmrose, et al., 2004)
The EDR membrane system is constructed the same as the electrodialysis process,
but the EDR system reverses direction of the charge and ion movement several times hourly. The
direction of the charge is changed frequently to reverse the electrical polarity and flush fouling
ions from the membrane. (Malmrose, et al., 2004)
The electricity cost to operate ED/ EDR may inhibit the use of this source water
treatment technique. ED and EDR are not common to large WTPs—those serving more than
10,000 people (U.S. EPA, 2008a).
6-11

Drinking Water Industry Report Section 6 – Source Water Treatment Technologies
6.4 ION EXCHANGE
In ion exchange, ions are transferred between different substances with an
exchange between solid and liquid being the most common. A precondition for using this
process is that the substances must be ionized when exchanged. In drinking water applications,
naturally occurring zeolites were first used for hardness removal, but modern ion exchange
technology uses resin materials that can be customized to remove specific contaminants of
interest. Ion exchange selectively removes a charged inorganic species (i.e., specific drinking
water contaminant) from the source water using an ion-specific resin (U.S. EPA, 1998).
Resins act as a repository of loosely held ions (cation or anion) that are exchanged
for like-charged ions that have a greater affinity for the resin than the currently held ions. Resins
are categorized as anion exchange or cation exchange resins. Anion exchange resins selectively
remove anionic species such as nitrate (NO
3
-
) and fluoride (F
-
). Cation exchange resins
selectively remove cationic species such as radium from the water and replace with protons (H
+
),
sodium ions (Na
+
), and potassium ions (K
+
). This process continues until all of the exchange
sites are used (i.e., saturated with the contaminant). (U.S. EPA, 1998)
Ion exchange often generates a backwash stream (or concentrate waste stream).
After the exchanger has exhausted all of the exchange sites, it must be regenerated or replaced.
Regeneration requires a reverse ion exchange and it is accomplished with a concentrated solution
of a common ion, usually a salt, so that the pH of the water is not affected. The contaminated
ions are exchanged for the concentrated salt common ions, and a waste stream requiring
treatment and/or disposal is created. Anion exchange resins may be regenerated using sodium
hydroxide or sodium chloride solutions by replacing the contaminant ions with a hydroxide
(OH
-
) or chloride (Cl
-
) ion (U.S. EPA, 1998). Cation exchange resins may be regenerated using
acid (i.e., replacing the contaminant with a proton, H
+
).
Some WTPs may not operate their ion exchange systems year-round. For
example, the Des Moines Water Works Fleur Drive Plant operates a lime softening system year-
6-12

Drinking Water Industry Report Section 6 – Source Water Treatment Technologies
round and adds nitrate removal through ion exchange during spring and summer months, when
nitrate concentrations are elevated in the river source water (U.S. EPA, 2006).
6.5 ADSORPTIVE MEDIA—ACTIVATED CARBON
While many adsorptive media are available, WTPs most commonly use activated
carbon. Plants use activated carbon filtration systems primarily to remove organic compounds
from source water. Organic compounds removed include those that may cause objectionable
taste or odor and those that pose potential negative health effects (e.g., pesticides). The most
common type of system is granular activated carbon (GAC), but WTPs also use powdered
activated carbon (PAC).
Activated carbon can adsorb ions or molecules on its surface from any
environmental media, with water and air being the most common. Activated carbon has a
random structure that is highly porous and exhibits different types of intramolecular forces.
Intramolecular attractions overcome the attractive forces of the liquid for the substance (i.e.,
source water contaminant), and the substance is deposited on the surface of the carbon. The large
surface area of activated carbon (1 gram = 1,000 square meters) allows removal of trace
quantities of contaminants from drinking water (ASCE/AWWA, 1997). Unlike filtration,
activated carbon plants do not remove the contaminants by straining; the removal is based on
adsorption rather than the size of the particulate. Detailed descriptions of the two activated
carbon forms follow.
GAC is a more coarse material than PAC and is usually employed later in the
treatment process to remove dissolved organic compounds as well as disinfection by-products.
GAC is usually used in a fixed bed, which water passes through for treatment. The carbon bed is
backwashed or surface washed to prevent buildup of solids and prevent fouling. As with other
treatment technologies, there is a finite amount of surface material that can adsorb impurities,
and when exceeded, “break through” occurs (i.e., contaminants are no longer removed from the
source water). At this point, the spent GAC must be replaced; the spent material may be
thermally reconditioned (regenerated) or discarded (U.S. EPA, 2000).
6-13

Drinking Water Industry Report Section 6 – Source Water Treatment Technologies
PAC is usually added early in the treatment process and is used to remove organic
contaminants that are associated with taste and odor. On a limited basis, PAC is also used to
remove seasonal contaminants like pesticides. PAC grains are 10 to 100 times smaller than GAC
grains. Use of PAC is less efficient than GAC due to less carbon material per unit volume
treated. The PAC is mixed with the water to create a suspension. The PAC continues along with
the treated water to sedimentation or filtration and becomes part of the residuals. Care must be
taken when using the small PAC particles so that they do not interfere with the application of
other treatment chemicals and treatment processes. For example, PAC can adsorb free and
combined chlorine, chlorine dioxide, and potassium permanganate, thereby reducing their
effectiveness (U.S. EPA, 1998).
6.6 DISINFECTION
Both surface and ground water sources typically require disinfection to eliminate
or inactivate microbiological populations. The application of disinfecting agents to a potable
water supply has been practiced for over a century and is recognized as one of the most
successful examples of public health protection. Historically, chlorine was the disinfectant used,
but more recently other chemicals such as chlorine dioxide, chloramines, and ozone have been
used to purify water. Non-chemical methods of disinfection include heat and radiation (e.g.,
ultraviolet light). The general disinfection reaction mechanism is chemical or physical
interference with the microorganism structure and cell membrane function.
If the microorganism cell membrane is compromised or penetrated, the
microorganism dies. Disinfection does not totally destroy pathogens, but eliminates the ability to
cause disease or interfere with normal body functions. The original disinfection theory, proposed
by Harriet Chick over 100 years ago, was that disinfection is a function of the concentration of
the treatment chemicals and the length of time they stay in contact with the pathogen (Chick,
1908). This concept of “CT values
19
” as a way to evaluate disinfection effectiveness continues
today. The lower the CT value, the more effective the disinfecting agent.
19
CT is the product of disinfectant residual concentration “C” in milligrams per liter (mg/L) and contact time “T” in
minutes to achieve a 3 log reduction of Giardia and a 4 log reduction of viruses (Chick, 1908).
6-14

Drinking Water Industry Report Section 6 – Source Water Treatment Technologies
WTPs perform two kinds of disinfection: 1) primary disinfection, and 2)
secondary disinfection. Primary disinfection achieves the desired level of microorganism kill or
inactivation. Secondary disinfection maintains a disinfectant residual in the finished drinking
water to prevent regrowth of microorganisms as water passes through the distribution system.
WTPs may use different chemicals for the two kinds of disinfection. Both kinds of disinfection
might affect chemicals in the residuals.
Primary disinfection occurs early in the source water treatment, prior to
sedimentation or filtration. Although no residuals are generated during this treatment step, the
disinfectant used (e.g., chlorine) or disinfection by-products may be present in the WTP residual
waste streams (e.g., filter backwash). Chlorine, ozone with another secondary disinfectant, and
UV light with another secondary disinfectant are effective primary disinfectants (National
Drinking Water Clearinghouse, 1996a).
Secondary disinfection occurs at the end of source water treatment, either at the
finished drinking water clear well or at various points in the distribution system. This
disinfection step is used to maintain a disinfectant residual in the finished drinking water to
prevent regrowth of microorganisms. The secondary disinfection process does not result in
residuals generation; however, water from the clear well may be used to backwash filters. As a
result, disinfectant added to the finished drinking water may become part of the filter backwash.
Chlorine and chloramines are effective secondary disinfectants (National Drinking Water
Clearinghouse, 1996a).
Almost all WTPs disinfect the source water prior to delivery—98 percent of the
ground water plants and 99 percent of the surface water plants (U.S. EPA, 2002b). The common
methods of disinfection are discussed in subsections below.
6.6.1 Disinfection with Chlorine (Chlorination)
When dissolved in water, chlorine gas quickly forms hypochlorous acid (HOCl),
which in turn, dissociates into hypochlorite ion (OCl
-
) (ASCE/AWWA, 1997). The hypochlorous
acid form of chlorine is a more effective disinfectant that the dissociated form, hypochlorite ion.
6-15

Drinking Water Industry Report Section 6 – Source Water Treatment Technologies
Chlorine gas, however, is toxic and has a density greater than air, therefore gas leaks accumulate
and present significant safety concerns. Properly engineered gas handling systems, continuous
training, or switching to a non-gaseous chlorine form like calcium hypochlorite reduce safety
concerns.
In the early 1970s, researchers discovered that the use of chlorine for disinfection
of drinking water produced microgram per liter (μg/L) quantities of halogenated methane
compounds (e.g., trihalomethane). The halogenated methane compounds, known as disinfection
by-products, are suspected to be carcinogens (Chlorine Chemistry Council, 2003). EPA limits
the amount of total trihalomethanes (TTHMs) in the drinking water to 0.08 mg/L (U.S. EPA,
2008b). The balance between producing microbiologically safe drinking water without long term
health effect implications from disinfection by-products became a major problem for some
systems. Alternatives to chlorine disinfection have been known for a long time, and the
discovery of halogenated methane compounds in chlorine-treated drinking water increased the
pressure to explore these alternatives.
6.6.2 Disinfection with Chlorine Dioxide
Chlorine dioxide has been used in some drinking water systems where an elevated
pH (>7) of the processed water has reduced the effectiveness of chlorine. Chlorine dioxide is
formed when chlorine (gaseous or liquid form) is mixed with sodium chlorite. As with chlorine,
WTPs must safely handle chlorine dioxide: it must be generated when used because it can not be
safely stored due to explosive characteristics. Also, reaction by-products or waste materials can
be toxic, such as chlorite (ClO
2,
MCL 1.0 mg/L) and chlorate (Cl
2
O
2
) ions (U.S. EPA, 2008b).
On the positive side, chlorine dioxide does not dissociate or disproportionate under normal
drinking water treatment conditions, is a strong oxidant, and does not form halogenated
disinfection by-products. It is sometimes used in conjunction with ozone systems as a residual
disinfectant.
6-16

Drinking Water Industry Report Section 6 – Source Water Treatment Technologies
6.6.3 Disinfection with Chloramines (Chloramination)
Chloramines (or combined residual chlorine) result when chlorine reacts with
ammonia. The ammonia can be natural or added to ensure the production of chloramines.
Chloramines have been demonstrated as disinfectants, but are not as effective as other germicidal
agents. The combined residual from chloramines lasts longer than chlorine residuals; therefore,
chloramines are typically used as secondary disinfectants. In addition, the use of chloramines for
disinfection results in very few disinfection by-products; however WTPs may need to
periodically switch to free chlorine for biofilm control in the water distribution system (U.S.
EPA, 1999d).
From EPA national estimates (see Section 3.3), EPA determined that 2,002 WTPs
perform primary disinfection. Approximately 80 percent of the WTPs disinfect with free
chlorine. 318 WTPs (or 16 percent) use chloramines for primary disinfection (see Appendix A).
6.6.4 Ozone Disinfection
Ozone (O
3
) is an energetic species generated by electrical discharge through dry
air or pure oxygen and tends to oxidize anything it contacts. Ozone disinfects microbes
effectively and can easily penetrate the sturdy cell membranes of protozoa like Cryptosporidium
(Tchobanoglous, et al., 2003). In addition to the on-site generation safety concerns, the main
concern with using ozone as a disinfectant is that its “half life” in water is only 30 minutes
(Lenntech, 2006). If ozone alone is used as the disinfectant in large distribution systems
(characterized by a residence time of 2 to 3 days), this residual concentration “half life” is
insufficient to maintain the microbiological integrity of the finished water. Use of ozone
disinfection at large drinking water systems requires booster ozone additions or supplemental
disinfection. Ozone disinfection is more commonly used to disinfect wastewater. EPA estimated
that 65 WTPs (only three percent) use ozone for primary disinfection (see Appendix A). Figure
6-3 shows an ozone disinfection process flow diagram.
6-17

Drinking Water Industry Report Section 6 – Source Water Treatment Technologies
Ozone Destruction
Ozone Generation
Ozone Contact Basin
Feed Gas Preparation
• Oxygen Production
• Oxygen Storage
• Air/Oxygen Treatment
Recycle
Off
-
Gases
Disinfected
Water
Raw Water In
Figure 6-3. Ozone Disinfection Process Flow Diagram (U.S. EPA, 1986)
6.6.5 Ultraviolet Light Disinfection
In ultraviolet (UV) disinfection, electromagnetic energy (UV radiation) is
transferred from a mercury arc lamp to an organism’s genetic material. The UV radiation
penetrates microorganism cell membranes and destroys the microorganisms’ ability to
reproduce. The application of UV disinfection for source water treatment is limited because
turbidity and suspended solids can render UV disinfection ineffective (U.S. EPA, 1999c). As
with ozone disinfection, UV disinfection requires large drinking water systems to add a
secondary disinfectant to maintain the microbiological integrity of the finished water.
6.7 OTHER CHEMICAL ADDITIONS
In addition to disinfection, coagulation, and precipitative softening chemicals,
drinking water systems add other chemicals to drinking water to control corrosion and scaling,
facilitate solids removal, adjust pH, and impart properties to the drinking water. The process of
adding the chemicals does not generate residuals; however, portions of the chemicals may
become part of the residuals at a downstream operation (e.g., sedimentation tank underflow).
6-18

Drinking Water Industry Report Section 6 – Source Water Treatment Technologies
6.7.1 Corrosion and Scale Control
To maintain pipes and tanks in the drinking water distribution system, systems
add chemicals to control corrosion (i.e., deterioration of material) and scale (i.e., film build-up).
Systems use chemicals such as phosphates and zinc for the control of scaling and corrosion.
Corrosion and scale control occurs at 26 percent of the ground water plants and 58 percent of the
surface water plants (U.S. EPA, 2002b).
Selected chemicals minimize scaling and corrosion by forming a protective film
that reduces the electrochemical reactions between the plumbing material and the water. pH
control with lime or strong bases, such as sodium hydroxide (NaOH) and potassium hydroxide
(KOH) contribute to the stability of the water and assist in reducing corrosion. About 45 different
blended phosphate chemicals listed in ANSI/NSF Standard 60 can be used for corrosion control
and 12 miscellaneous zinc products can be custom blended to serve different water quality
conditions. Zinc does not have a primary standard (no MCL), but does have a secondary standard
of 5 mg/L (U.S. EPA, 2008b). ANSI/NSF Standard 60 recommends that zinc not exceed 2 mg/L
in the finished water.
6.7.2 Solids Removal Using Sequestering Agents
Iron and manganese are metals found in many drinking water supplies, especially
ground water. EPA has secondary standards for iron (0.3 mg/L) and manganese (0.05 mg/L)
(U.S. EPA, 2008b) and both metals can cause off-tastes and staining of customer sinks. If iron
and manganese concentrations are low, the aesthetic problems can be addressed by adding a
sequestering agent that will tie up the soluble form of the metal and inhibit precipitation
(staining). Blended phosphates, sodium silicate, and sodium polyphosphate are sequestering
agents listed in ASNI/NSF Standard 60.
The use of sequestering agents occurs at 45 percent of the ground water plants and
32 percent of the surface water plants (U.S. EPA, 2002b).
6-19

Drinking Water Industry Report Section 6 – Source Water Treatment Technologies
6.7.3 pH Adjustment
Adjusting the pH of a drinking water treatment process is often necessary to
ensure the proper interactions between chemicals and contaminants. WTPs add lime or a strong
base such as sodium hydroxide or potassium hydroxide to raise the pH. Sodium hydroxide is
listed in ANSI/NSF Standard 60. In cases where the pH must be lowered, plants use carbon
dioxide or purified mineral acids, such as hydrochloric acid (HCl).
6.7.4 Water Additives
Small amounts of fluoride (~1.0 mg/L) in the drinking water can play a significant
role in reducing tooth decay. Sodium fluoride, sodium fluorosilicate, and fluorosilicic acid are
used by the drinking water systems and all three are covered by AWWA Standards. Fluoridation
is used by 21 percent of the ground water plants and 49 percent of the surface water plants (U.S.
EPA, 2002b).
6.8 REFERENCES
American National Standards Institute (ANSI)/National Sanitation Foundation (NSF) Standard
60, 2007. Drinking water Treatment Chemicals-Health Effects, Purchased from www.nsf.org.
Document Control Number (DCN) DW00960.
American Society of Civil Engineers (ASCE)/American Water Works Association (AWWA),
1997. Water Treatment Plant Design, 3
rd
Edition. New York: McGraw-Hill. DCN DW00961.
Chick, H., 1908. “An Investigation of the Laws of Disinfection,” Journal of Hygiene, 8:92. DCN
DW00964.
Chlorine Chemistry Council, 2003. Drinking Water Chlorination: A Review of Disinfection
Practices and Issues, Arlington, VA, February 2003. DCN DW00651.
Lenntech, 2006. FAQs Ozone. Retrieved from http://www.lenntech.com/faqozone.htm. DCN
DW00639.
Malmrose, et al., 2003. Paul Malmrose, Jim Lozier, Jason Marie, Michael Mickley, Robert Reiss,
Jerry Russell, James Schaefer, Sandeep Sethi, Jennifer Worley, Residual Management Research
Committee Subcommittee on Membrane Residual Management, “2003 Committee Report:
Residuals Management for Low-Pressure Membranes,” Jour. AWWA, 95:6:68. AWWA, June
2003. DCN DW00003.
6-20

Drinking Water Industry Report Section 6 – Source Water Treatment Technologies
Malmrose, et al., 2004. Paul Malmrose, Jim Lozier, Michael Mickley, Robert Reiss, Jerry
Russell, James Schaefer, Sandeep Sethi, Jennifer Manuszak, Robert Bergman, and Khalil Z.
Atasi, Residual Management Research Committee Subcommittee on Membrane Residual
Management, “2004 Committee Report: Residuals Management for Desalting Membranes,”
Jour. AWWA, 96:12:73. AWWA, December 2004. DCN DW00032.
Manahan, Stanley E., 1993. Section 13.7. “Removal of Calcium and Other Metals.” In
Fundamentals of Environmental Chemistry, pages 463-464. Chelsea, MI: Lewis Publishers.
DCN DW03757.
The Merit Partnership, 2002. Reverse Osmosis Applications for Metal Finishing Operations.
Merit Partnership P2 Project for Metal Finishers. January 2002. DCN DW003774.
National Drinking Water Clearinghouse, 1996a. Tech Brief: Disinfection (#DWBRPE47), June
1996. DCN DW03758.
National Drinking Water Clearinghouse, 1996b. Tech Brief: Filtration (#DWBRPE50),
September 1996. DCN DW00874.
Tchobanoglous, et al., 2003. George Tchobanoglous, Franklin L. Burton, H. David Stensel,
Wastewater Engineering Treatment & Reuse, 4
th
edition. Metcalf & Eddy, Inc., New York:
McGraw-Hill. DCN DW00871.
U.S. Environmental Protection Agency (EPA), 1986. Design Manual: Municipal Wastewater
Disinfection (EPA 625-1-86-021), Office of Research and Development, Cincinnati, Ohio.
U.S. EPA, 1998. Small System Compliance Technology List for the Non-Microbial
Contaminants Regulated Before 1996 (EPA 815-R-98-002), Office of Water, Washington, DC.
DCN DW00883.
U.S. EPA, 1999c. Wastewater Technology Fact Sheet: Ultraviolet Disinfection (EPA 832-F-99-
064), Office of Water Municipal Technology Branch, Washington, DC. DCN DW00677.
U.S. EPA, 1999d. Alternative Disinfectants and Oxidants Guidance Manual (EPA 815-R-99-
014), Office of Water, Washington, DC. DCN DW00647.
U.S. EPA, 2000. Wastewater Technology Fact Sheet: Granular Activated Carbon Adsorption and
Regeneration (EPA 832-F-00-017), Office of Water, Washington, DC. DCN DW00650.
U.S. EPA, 2002a. Filter Backwash Recycling Rule: Technical Guidance Manual (EPA 816-R-
02-014), Office of Ground Water and Drinking Water, Washington, DC. DCN DW00064.
U.S. EPA, 2002b. Community Water System Survey 2000 (EPA 815-R-02-005), Office of Water,
Washington, DC. DCN DW00001.
U.S. EPA, 2006. Drinking Water Treatment Plant Site Visit Report: Des Moines Water Works
Fleur Drive Water Treatment Plant, Office of Water, Washington, DC. DCN DW00918.
6-21

Drinking Water Industry Report Section 6 – Source Water Treatment Technologies
U.S. EPA, 2008b. National Primary Drinking Water Standards (List of Drinking Water
Contaminants and MCLs) (original document published in 2003, DCN DW00657), Office of
Ground Water and Drinking Water, Washington, DC. Retrieved from
http://www.epa.gov/safewater/contaminants/index.html Accessed April 2008 for updates.
U.S. EPA, 2009. Drinking Water Survey Response Database – Technical Data for the 2006
Drinking Water Industry Questionnaire. Office of Water, Washington, DC. DCN DW03790.
U.S. Geological Survey (USGS), 2007. “Saline water,” Water Science for Schools. Retrieved
July 2007, from http://ga.water.usgs.gov/edu/saline.html. DCN DW03761.
6-22

SECTION 7
TYPES OF RESIDUALS PRODUCED BY SOURCE WATER
TREATMENT
The previous section discusses the treatment processes and common treatment
trains that are used at water treatment plants (WTPs) to produce drinking water. The treatment
processes used to produce drinking water may generate waste streams (or residuals) that the
WTP must manage. Two of the treatment processes presented in the previous section,
disinfection and other chemical addition, may contribute chemicals to the residuals, but do not
generate waste streams themselves. Therefore these two processes are not specifically discussed
in this section. This section discusses residuals generated by the following water treatment
processes:
• Presedimentation (Section 7.1);
• Coagulation, flocculation, and sedimentation (Section 7.2);
• Precipitative softening (Section 7.3);
• Filtration, microfiltration, and ultrafiltration (Section 7.4);
• Membrane desalination (Section 7.5);
• Ion exchange (Section 7.6); and
• Activated carbon (adsorption process) (Section 7.7).
Water treatment plants (WTPs) may use more than one of the treatment processes
listed above and may generate multiple types of residuals. The volume and characterization of
the residuals generated depends on the quality of the source water, the drinking water production
rate, efficiency of the source water treatment system, the amount of treatment chemical used, and
type of source water treatment. The residuals volume at a WTP may vary seasonally or monthly
(U.S. EPA, 1993). EPA collected data through literature searches, EPA and state sources, and the
2006 industry questionnaire to quantify residuals generation rates and composition before
residuals treatment. An overview of the data sources is presented in Section 2 of this document.
7.1 PRESEDIMENTATION
As discussed in Section 6, presedimentation is a sedimentation basin operated at
the head of the WTP. Presedimentation uses gravity to remove suspended solids from source
7-1

Drinking Water Industry Report Section 7 – Types of Residuals Produced by Source Water Treatment
water. The residence time, which depends on the WTP design, capacity, and production rate, is
an important factor in the efficiency of solids separation and removal. Clay and organics settle
slowly and are not removed during presedimentation; these contaminants require coagulants to
assist settling. Silt also has a slow settling velocity; 2-micron silt particles settle at a rate of 10
millimeters per hour (0.4 inches per hour). Sand and grit settle more rapidly; 600-micron sand
particles settle at a rate of 900 meters per hour (50 feet per minute) (New Zealand Ministry of
Health, 2005). If the presedimentation basin has a residence time of two days, then all of the sand
and grit will be in the sludge, but very little of the clay and silt. The composition of the solids in
the sludge is site-specific. Depending on the composition, settling basins can remove between 50
and 90 percent of the influent solids (U.S. EPA, 1999). Following presedimentation, WTPs
remove smaller particles during the coagulation, flocculation, sedimentation, and filtration
processes, as shown in Figure 7-1.
Raw
Water
Grit
Pre-Sedimentation
Waste
Pre-Sedimentation Basin Rapid Mix
Coagulant Sludge
Filter Backwash
Waste
Finished
Water
Flocculation Final Sedimentation Basin Filters
Figure 7-1. Residuals from Source Water Solids Removal (U.S. EPA/ACSE/AWWA, 1996)
7.2 RESIDUALS FROM COAGULATION, FLOCCULATION, AND
SEDIMENTATION
During coagulation, flocculation, and sedimentation, solids settle to the bottom of
clarifiers and sedimentation basins. Coagulation is the addition of chemical agent(s) to the solids
settling process to reduce the negative surface charges by introducing positive ions, which allows
the particulates to agglomerate and settle. Aluminum and iron salts are common coagulant aids
whose positive trivalent forms are insoluble at normal conditions for drinking water treatment
and precipitate along with the neutralized suspended solids (Tchobanoglous, et al., 2003). The
charge neutralization reactions begin immediately, necessitating a rapid mix chamber.
7-2

Drinking Water Industry Report Section 7 – Types of Residuals Produced by Source Water Treatment
The term flocculation is the agglomeration of small finely separated particles into
larger particles that become heavier than water. Optimum floc formation is best carried out under
conditions of gradually reducing energy: turbulent rapid mix is reduced to gentle agitation, which
is further reduced to quiescent deposition.
Within sedimentation basins, solids settle by gravity to the bottom. The underflow
sludge is removed from the basin on either a continuous or batch basis. In continuous sludge
removal, rakes or blades push the sludge along the bottom of the settling basin to an outlet. In
batch removal, basins are drained and the sludge is removed with the remaining basin water and
cleaning water. Batch removal occurs when the settling volume in the basin is no longer effective
(i.e., sludge displaces too much settling volume). The time between batch removals varies from a
few weeks to over a year.
The volume of coagulation sludge generated depends on the plant production,
amount of coagulant or other treatment chemical added (dose), and amount of suspended solids
in the source water. Table 7-1 presents typical coagulation sludge volumes generated (U.S. EPA,
1993). The characteristics of coagulation sludge vary depending on initial water quality and the
amount and type of coagulant used (e.g., higher aluminum concentration in the sludge using
aluminum-based coagulant). Coagulation sludge predominately contains the coagulant metal
hydroxides along with source water natural organic matter, suspended solids, microorganisms,
radionuclides, and other organic and inorganic constituents. The metals found in coagulation
sludge include aluminum, arsenic, and occasionally cadmium, chromium, copper, iron, lead,
manganese, nickel, and zinc (Cornwell, 1999).
7-3

Drinking Water Industry Report Section 7 – Types of Residuals Produced by Source Water Treatment
Table 7-1. Typical Chemical Coagulation Sludge Volumes
Population Served
Range
Average Water
Treatment Plant
Flow (MGD)
Water Treatment
Plant Design Flow
(MGD)
Typical Sludge
Volume Range
(GPD)
Average Sludge
Volume
(GPD)
1,001 to 3,300
0.23
0.7
7 – 2,600
770
3,301 to 10,000
0.7
1.8
18 – 6,700
2,000
10,001 to 25,000
2.1
4.8
48 – 17,800
5,300
25,001 to 50,000
5
11
110 – 40,900
12,100
50,001 to 75,000
8.8
18
180 – 66,800
19,800
75,001 to 100,000
13
26
260 – 96,600
28,600
100,001 to 500,000
27
51
510 – 189,400
56,200
500,001 to 1,000,000
120
210
2,100 – 779,900
231,300
Greater than 1,000,000
270
430
4,300 – 1,596,900
473,500
Source: U.S. EPA, 1993.
MGD – Million gallons per day.
GPD – Gallons per day.
If the source water has a high concentration of total suspended solids (TSS), then
the coagulant sludge will contain a high percentage of gelatinous, hydroxide precipitates. The
alum and ferric (or iron) sludge exhibit poor compaction traits, ranging from 0.5 to 2 percent
solids (ASCE/AWWA, 1997). Consequently, coagulation sludge usually requires additional
processing such as thickening, dewatering, or drying prior to disposal. Because of their low
solids content, these sludges are difficult to dewater. They are also biologically inert with little
organic content and have little value as a fertilizer or soil conditioner. Section 11 discusses
residuals treatment and management practices.
7.3 RESIDUALS FROM PRECIPITATIVE (LIME) SOFTENING
WTPs use precipitative softening to remove divalent ions in water, particularly
calcium and magnesium, by the addition of lime. The concentration of the divalent ions in the
water is often referred to as the water’s “hardness.” The lime increases the pH and reacts with the
ions to form a precipitate of insoluble calcium carbonate and magnesium hydroxide. Softening
sludge (or carbonate residuals) settles to a solids content ranging from 2 to 15 percent
(ASCE/AWWA, 1997). Softening sludge is easier to dewater and compact than coagulation
sludge (see Section 7.2). Table 7-2 presents typical lime softening sludge volumes produced by
WTPs (U.S. EPA, 1993).
7-4

Drinking Water Industry Report Section 7 – Types of Residuals Produced by Source Water Treatment
Table 7-2. Typical Lime Softening Sludge Volumes
Population Served
Range
Average Water
Treatment Plant
Flow (MGD)
Water Treatment
Plant Design Flow
(MGD)
Typical Sludge Volume
Range
(GPD)
Average Sludge
Volume
(GPD)
1,001 to 3,300
0.23
0.7
2,800 – 10,700
8,500
3,301 to 10,000
0.7
1.8
7,200 – 27,400
21,900
10,001 to 25,000
2.1
4.8
19,300 – 73,100
58,300
25,001 to 50,000
5
11
44,200 – 167,500
133,600
50,001 to 75,000
8.8
18
72,300 – 274,100
218,600
75,001 to 100,000
13
26
104,400 – 395,900
315,800
100,001 to 500,000
27
51
204,800 – 776,600
619,400
500,001 to 1,000,000
120
210
843,400 – 3,198,000
2,550,600
Greater than 1,000,000
270
430
1,726,900 – 6,548,200
5,222,700
Source: U.S. EPA, 1993.
MGD – Million gallons per day.
GPD – Gallons per day.
Softening sludge is biologically inert and has a high pH (typically greater than
10.5) due to unreacted lime and high alkalinity. The sludge contains calcium carbonate,
magnesium hydroxide, other divalent ions, natural organic matter from the source water,
inorganics, suspended solids, microorganisms, and radionuclides. Metals found in the softening
sludges include calcium, magnesium, arsenic, barium, cadmium, chromium, lead, mercury,
selenium, and silver (Cornwell, 1999).
In lime softening, sludge generation rates depend on the ratio of calcium
carbonate to magnesium hydroxide and the type of clarifier/sedimentation basin. Conventional
gravity sedimentation basins generate sludge with solids concentrations of only 2 to 4 percent,
whereas, sludge blanket clarifiers generate sludge with solids concentrations up to 30 percent
(U.S. EPA/ASCE/AWWA, 1996).
Softening sludges are generally dense, stable, and inert materials that dewater
easily to a solids content up to 50 to 60 percent. However, if the hardness is due to magnesium,
the hydroxide sludge is more difficult to handle and dewater (Cornwell, 1999). Figure 7-2
presents the sources of residuals from a typical precipitative softening plant.
7-5

Drinking Water Industry Report Section 7 – Types of Residuals Produced by Source Water Treatment
Raw
Water
Grit
Pre-Sedimentation
Waste
Pre-Sedimentation Basin Rapid Mix
Lime Softening
Sludge
Filter Backwash
Waste
Finished
Water
Flocculation Final Sedimentation Basin Filters
Figure 7-2. Residuals from Precipitative Softening WTP
7.4 RESIDUALS FROM FILTRATION
WTPs use filtration to remove finer particles and metals. At some WTPs,
filtration is the only solids removal step. Filter types include non-membrane filters such as multi-
media, slow sand, and diatomaceous earth and low-pressure membranes such as microfiltration
(MF) and ultrafiltration (UF).
7.4.1 Filters (non-membrane)
Filtration removes suspended material in the source water by allowing water to
pass through the filter media while suspended solids accumulate in the interstices of the filter
media. As the filter run continues and more particles are removed, it becomes more difficult for
the inlet water to easily make its way through the filter. This condition is called filter head loss
and is an indication of waning filter performance. At a predetermined filter head loss value, the
filter is taken out of service for backwash.
Backwashing is the process of using finished water to reversely expel the particles
collected on the filter media. The plant collects the filter backwash water (containing the
particles) in an area separated from the filter inlet. Due to the relatively low level of filtered
particles and the relatively large volume of water necessary to clean the filter, the resulting
backwash residuals water is dilute (50 to 400 mg/L of suspended solids) and difficult to dewater
(U.S. EPA/ASCE/AWWA, 1996).
7-6

Drinking Water Industry Report Section 7 – Types of Residuals Produced by Source Water Treatment
Filter backwash contains particulates including clay and silt particles,
microorganisms (bacteria, viruses, and protozoan cysts), colloidal and precipitated humic
substances, and other natural organic particulates from the decay of vegetation. At conventional
and direct filtration plants, filter backwash also contains precipitates of aluminum or iron used in
coagulation (Cornwell, 1999).
The volume of filter backwash wastewater generated depends on the number of
filters, frequency of backwash, and duration of backwash events. The volume is typically
between 2 and 5 percent of the finished water produced (U.S. EPA/ASCE/AWWA, 1996). This
is a sizeable residuals volume. Consequently, many WTPs employ a flow equalization system to
settle and remove some of the solids and recycle the backwash water to the head of the source
water treatment plant.
After backwashing, WTPs may wash the filter to ensure adequate filter
performance. The spent wash water is called “filter-to-waste.” By generating the filter-to-waste
stream, WTPs can check the effluent quality from the filter prior to bringing the filter back on-
line. Filter-to-waste is the filter effluent for the first 15 to 60 minutes after startup (following
backwash). The filter-to-waste stream is equalized and returned to the head of the treatment
plant, rather than distributed to customers (U.S. EPA/ASCE/AWWA, 1996).
7.4.2 Low-Pressure Membranes
Low-pressure membranes (MF/UF) generate filter backwash waste streams,
similar to other filtration processes, and spent chemical cleaning solutions. MF and UF systems
remove suspended solids, turbidity, inorganic and organic colloids, microorganisms (protozoan
cysts and bacteria), viruses (UF only), and some organic fractions (UF only) from the source
water. The volume of backwash generated is typically between 2 and 15 percent of the plant flow
rate. The backwash stream represents the majority of residuals generated from MF/UF treatment
process (95 to 99 percent of the total volume of residuals). The remaining 1 to 5 percent of
membrane residuals is generated by chemical cleaning procedures (Malmrose, et al., 2003).
7-7

Drinking Water Industry Report Section 7 – Types of Residuals Produced by Source Water Treatment
Table 7-3 presents typical characteristics of low-pressure membrane backwash
residuals. These characteristics vary with feed flow rate and backwash frequency. (Malmrose, et
al., 2003)
Table 7-3. Typical Characteristics of Low-Pressure Membrane Backwash Residuals
Frequency of application
Every 10 to 60 minutes.
Volume of backwash
residuals generated/
waste produced
2 to 15% of plant feed flow rate for recoveries of 85 to 98%.
(Daily chemically-
enhanced backwash (CEBW) wastes might be 0.2 to 0.4% of plant
feed flow rate.)
Characteristics of
backwash residuals
Algae, precipitated solids, possible chemical residues if using CEBW
Total organic carbon (TOC) concentration of 1 to 2 times the feed water
concentration (if no coagulant or absorbent is used).
If coagulant is used, the TOC could be 5 times the feed water concentration.
For recoveries of 85 to 98%, backwash will have a concentration factor of 7 to 50
times the feed water for total suspended solids (TSS) and Cryptosporidium.
If using CEBW (with chlorine, acid, or base), pH may be <6 or >9, and chlorine
residual may be up to 1,000 mg/L as Cl
2
.
Source: Malmrose, et al., 2003.
Some systems use coagulants, powdered activated carbon (PAC), or other
chemicals (e.g., potassium permanganate) as pretreatment to membrane filtration to remove
some solids prior to the membrane. This pretreatment helps to reduce fouling of the membrane
and reduce the backwash frequency. The characteristics of the resulting residuals from these
pretreatment operations closely resemble those of coagulation sludge (Malmrose, et al., 2003).
MF/UF systems also generate spent chemical cleaning solution residuals during
the membrane cleaning processes used to control fouling (CEBW and clean in place (CIP)).
Cleaning solution residuals reflect the chemicals used in the cleaning process. Only a portion of
the active chemical ingredient is consumed during the cleaning process, so the resulting chemical
cleaning waste includes some remaining active chemical ingredient, as well as salts from
chemical reactions between the chemicals and foulants, dissolved organic materials, and
suspended solids. While some plants refresh the active ingredient in spent cleaning solutions and
reuse it to minimize waste quantities, this practice can result in more concentrated waste cleaning
solutions. Table 7-4 summarizes the characteristics of some typical waste chemical cleaning
solutions (Malmrose, et al., 2003).
7-8

Drinking Water Industry Report Section 7 – Types of Residuals Produced by Source Water Treatment
Table 7-4. Typical Characteristics of Spent Low-Pressure Membrane Chemical
Cleaning Solutions
Frequency of application
Daily to once every 3 or 4 months.
Volume of residuals
generated
Monthly CIP wastes normally <0.05% of plant feed flow rate.
Daily CEBW wastes might be 0.2 to 0.4% of plant feed flow rate.
Chemicals commonly
used
Sodium hypochlorite – 500 to 1,000 mg/L as Cl
2
.
Citric or hydrochloric acid – pH 1 to 2.
Caustic soda – pH 12 to 13.
Surfactant – 0.1% by weight.
Characteristics of spent
cleaning solutions
pH from 2 to 14.
Chlorine residual up to 1,000 mg/L as Cl
2
.
Low concentrations of surfactants.
TSS up to 500 mg/L (neutralization may precipitate additional solids).
TOC 10 to 30 times the feed water concentration.
5-day biochemical oxygen demand (BOD
5
) up to 5,000 or 10,000 mg/L (if citric acid
is used).
Source: Malmrose, et al., 2003.
7.5 RESIDUALS FROM MEMBRANE DESALINATION
As discussed in Section 6 of this document, membrane desalination technologies
include reverse osmosis (RO), nanofiltration (NF), electrodialysis (ED), and electrodialysis
reversal (EDR). Membranes are typically used to remove dissolved solids and ions. In addition to
dissolved solids and ions, membranes can also remove dissolved organics, dissolved gases,
biological contaminants, and suspended solids (U.S. EPA/ASCE/AWWA, 1996). However,
industry practice is to remove biological material and particulates via pretreatment. Plants
typically pretreat the source water prior to membrane desalination to protect and extend the life
of the membrane. Pretreatment steps commonly include:
• Acid addition – lowering pH to between 5.5 and 7.0.
• Anti-scalant addition – to prevent membrane fouling.
• Filtration – remove suspended particles.
The filtration step generates a backwash waste stream. Figure 7-3 presents typical
residuals generated from membrane desalination plants.
7-9

Drinking Water Industry Report Section 7 – Types of Residuals Produced by Source Water Treatment
Membrane
Pretreatment
Raw Water
Finished Water
(Permeate)
Pretreatment Residuals
(e.g., filter backwash)
Concentrate
Cleaning Waste
(intermittent)
Figure 7-3. Residuals from Membrane Desalination
Membrane desalination systems generate a clean permeate stream that passes
through the membranes and a reject stream (or concentrate) containing the contaminants that are
retained by the membranes for separate disposal. The types of contaminants in the concentrate
are generally the same as those in the source water (i.e., very few process-added chemicals).
Contaminant concentrations in the concentrate are typically 4 to 10 times feed
water concentrations and depend upon the rejection characteristics of the membrane and finished
drinking water (i.e., permeate) production. If pretreatment is used, then the feed water to the
desalting membranes will have lower levels of certain constituents and particles; however, feed
water levels of other constituents may increase. For example, coagulation pretreatment will
increase the inorganic ions, such as sulfate, iron, and aluminum, and polymer or sulfuric acid
pretreatment may increase residual organics. Table 7-5 lists the target contaminants typically
removed by membrane desalination (Malmrose, et al., 2004).
The rejection rate for a contaminant is the percentage of the contaminant in the
source water that does not pass through the membrane, but becomes part of the concentrate
stream. The rejection rate depends on the contaminant size and interaction with the membrane. In
general, the larger the pore size of the membrane, the lower the rejection rate. RO systems have
rejection rates from 90 to 99.8 percent for monovalent ions and from 98 to 99.9 percent for
divalent ions (e.g., hardness) (Malmrose, et al., 2004). NF membranes have rejection rates from
40 to 90 percent for monovalent ions and from 80 to 98 percent for divalent ions (Malmrose, et
al., 2004). The ED/EDR process can reject more than 90 percent of dissolved ions (U.S.
EPA/ASCE/AWWA, 1996).
7-10

Drinking Water Industry Report Section 7 – Types of Residuals Produced by Source Water Treatment
Table 7-5. Membrane Desalination: Typical Target Contaminants by Source Water
Source Water
Typical Target Contaminants
Surface water
Total Organic Carbon (TOC)
Disinfection By-product (DBP) Precursors
Microorganisms (or pathogens)
Pesticides / Synthetic Organic Compounds (SOCs)
Taste & Odor Compounds
Ground water
Hardness
Color (indicative of dissolved organic material)
TOC
Inorganic and organic compounds / chemicals
Brackish surface water and ground water
Total Dissolved Solids (TDS)
Hardness
Chloride and sodium
Seawater
TDS
Chloride and Sodium
Bromide
Boron
Source: Malmrose, et al., 2004.
Table 7-6 presents typical design parameters for RO and NF membrane
desalination treatment plants (Malmrose, et al., 2004). The water recovery rate for a membrane is
the percentage of the feed water that passes through the membrane as permeate (finished water).
Table 7-6. Typical Membrane Desalination System (RO and NF) Design Parameters
Parameter
Surface Water
Fresh Ground
Water
Brackish Ground
Water
Seawater
Feed total dissolved solids
(TDS) (mg/L)
200-400
400-500
500-10,000
30,000-40,000
Water recovery
(% of feed)
80-90
80-90
65-85
40-60
Concentrate quantity
(% of feed)
10-20
10-20
15-35
40-60
Concentrate TDS (mg/L)
(at example recovery)
1,330-2,660
(85%)
2,660-3,330
(85%)
2,000-40,000
(75%)
60,000-80,000
(50%)
Concentration factor
a
5-10
5-10
2.9-6.7
1.7-2.5
Source: Malmrose, et al., 2004.
a – Ratio of total dissolved solids in concentrate to total dissolved solids in feed, assuming 100 percent salt rejection.
mg/L – Milligrams per liter.
7-11

Drinking Water Industry Report Section 7 – Types of Residuals Produced by Source Water Treatment
Membrane desalination plants also clean the equipment and generate spent
cleaning solutions every three to 12 months. Typically, the waste cleaning solution volume
generated during a clean in place of NF and RO is about 3 gallons per 100 square feet (1.2
liters/square meter). Typical waste cleaning solution volume is estimated by adding the total
empty vessel volume and pipe volume. In addition to spent cleaning solution, the plant may
generate one to two volumes of rinse water. Typical cleaning solutions, which may be diluted
with rinse water (feed or permeate), for NF and RO systems include acid (mineral or citric) to
remove inorganic contaminants and alkaline solutions (e.g., caustic soda with detergents or
surfactants) to remove organic contaminants and biofilms.
20
ED/EDR system cleaning solutions
typically include concentrated hydrochloric acid and sodium chlorine solutions. Occasionally,
chlorine solutions may be used to clean ED/EDR systems for organic contaminant and biofilm
removal. The waste cleaning solution volume is extremely small compared to treated flow (<0.1
percent) (Malmrose, et. al., 2004).
ED/EDR systems also produce a low flow waste stream called “electrode waste,”
which contains significant levels of hydrogen and chlorine gases that are typically stripped from
the electrode waste stream using a degasifier (which is part of the EDR system) (Malmrose, et
al., 2004).
7.6 RESIDUALS FROM ION EXCHANGE
Ion exchange may be used by WTPs to reduce hardness by replacing calcium and
magnesium ions in source water with sodium ions that are contained in the ion exchange resin.
Ion exchange can also remove nitrates, barium, radium, arsenate, selenate, excess levels of
fluoride, lead, and chromate (U.S. EPA/ASCE/AWWA, 1996). Once all the ion exchange sites
reach capacity, the plant must regenerate the ion exchange material, thus producing waste
concentrate that contains the source water contaminants. In addition to the waste concentrate, the
ion exchange process also generates backwash water and rinse water that is used before and after
the regeneration of the ion exchange resin, respectively. Waste concentrate generation rates from
ion exchange for water softening ranges from 1.5 to 10 percent of the water softened (U.S.
20
Biofilms are an accumulated mixture of microorganisms, organic contaminants, and inorganic contaminants.
7-12

Drinking Water Industry Report Section 7 – Types of Residuals Produced by Source Water Treatment
EPA/ASCE/AWWA, 1996). Table 7-7 presents typical ion exchange concentrate generation
rates for WTPs (U.S. EPA, 1993).
Table 7-7. Typical Ion Exchange Concentrate Volumes
Population Served
Range
Average Water
Treatment Plant Flow
(MGD)
Water Treatment Plant
Design Flow (MGD)
Range of Typical Concentrate
Generation Rates (GPD)
1,001 to 3,300
0.23
0.7
12,300 – 63,200
3,301 to 10,000
0.7
1.8
31,500 – 162,500
10,001 to 25,000
2.1
4.8
84,000 – 433,300
25,001 to 50,000
5
11
192,500 – 993,000
50,001 to 75,000
8.8
18
315,000 – 1,624,900
75,001 to 100,000
13
26
455,000 – 2,347,100
100,001 to 500,000
27
51
892,600 – 4,604,000
500,001 to 1,000,000
120
210
3,675,200 – 18,957,700
Greater than 1,000,000
270
430
7,525,400 – 38,818,100
Source: U.S. EPA, 1993.
MGD – Million gallons per day.
GPD – Gallons per day.
Table 7-8 lists typical concentrations of ions in ion exchange waste concentrate
(U.S. EPA/ASCE/AWWA, 1996).
Table 7-8. Typical Chemical Concentrations in Ion Exchange Waste Concentrate
Constituent
Average Concentration Range (mg/L)
Total dissolved solids
15,000 – 35,000
Calcium (Ca++)
3,000 – 6,000
Magnesium (Mg++)
1,000 – 2,000
Hardness (as CaCO3)
11,600 – 23,000
Sodium (Na+)
2,000 – 5,000
Chlorine (Cl-)
9,000 – 22,000
Source: U.S. EPA, ASCE, AWWA, 1996.
7.7 RESIDUALS FROM ADSORPTION (ACTIVATED CARBON)
Adsorption removes ions or molecules from the source water by adsorbing the
chemicals in the source water onto the treatment media. Adsorption is used to remove naturally
occurring organic materials, taste, odor, synthetic organic compounds, as well as disinfection by-
7-13

Drinking Water Industry Report Section 7 – Types of Residuals Produced by Source Water Treatment
products. Adsorption can use different types of adsorptive media, and the most common is
granular activated carbon (GAC). Residuals generated by GAC include backwash water (or
surface wash water) and spent media.
As the treatment process goes on, adsorption sites become filled. Once all the
adsorption sites are filled, breakthrough of the contaminant occurs (i.e., pollutants are no longer
removed from the influent but continue through to the GAC filter effluent). WTPs then perform
backwashing of the GAC filter bed. The time it takes for breakthrough to occur depends on the
concentration of the pollutant contaminants being removed.
Plants perform backwashing to disengage solids that have been entrapped in the
filter bed. Backwashing of GAC filter-adsorbers is essential to remove solids and to maintain the
desired hydraulic properties of the bed. Backwash water generally contains the removed
contaminants such as suspended solids, biological films, organics, and some filter media. The
volume and quantity of the GAC backwash stream depends on the influent source water quality.
The spent media (or carbon) is sent off site for regeneration or disposal.
Regeneration of the spent carbon is accomplished by thermal means (e.g., rotary kiln, hearth
furnace) and does not generate a wastewater stream.
7.8 REFERENCES
American Society of Civil Engineers (ASCE)/American Water Works Association (AWWA),
1997. Water Treatment Plant Design, 3
rd
Edition. New York: McGraw Hill. Document Control
Number (DCN) DW00961.
Cornwell, D.A., 1999. Water Treatment Plant Residual Management, In Water Quality and
Treatment: A Handbook of Community Water Supplies, 5
th
edition. New York: McGraw Hill.
DCN DW00247.
Malmrose, et al., 2003. Paul Malmrose, Jim Lozier, Jason Marie, Michael Mickley, Robert Reiss,
Jerry Russell, James Schaefer, Sandeep Sethi, Jennifer Worley, Residual Management Research
Committee Subcommittee on Membrane Residual Management, “2003 Committee Report:
Residuals Management for Low-Pressure Membranes,” Jour. AWWA, 95:6:68. AWWA, June
2003. DCN DW00003.
7-14

Drinking Water Industry Report Section 7 – Types of Residuals Produced by Source Water Treatment
Malmrose, et al., 2004. Paul Malmrose, Jim Lozier, Michael Mickley, Robert Reiss, Jerry
Russell, James Schaefer, Sandeep Sethi, Jennifer Manuszak, Robert Bergman, and Khalil Z.
Atasi, Residual Management Research Committee Subcommittee on Membrane Residual
Management, “2004 Committee Report: Residuals Management for Desalting Membranes,”
Jour. AWWA, 96:12:73. AWWA, December 2004. DCN DW00032.
New Zealand Ministry of Health, 2005. Draft Guidelines for Drinking Water Quality
Management for New Zealand, Chapter 12, October 2005. Retrieved from
www.moh.govt.nz/moh.nsf. DCN DW00930.
Tchobanoglous, et al., 2003. George Tchobanoglous, Franklin L. Burton, H. David Stensel,
Wastewater Engineering Treatment & Reuse, 4
th
edition. Metcalf & Eddy, Inc., New York:
McGraw-Hill. DCN DW00871.
U.S. Environmental Protection Agency (EPA), 1993. Large Water System Byproducts Treatment
and Disposal Cost Document (EPA 811-D-93-002), Office of Water, Washington, DC. DCN
DW00058.
U.S. EPA, 1999. Storm Water Technology Fact Sheet: Wet Detention Ponds (EPA 832-F-99-
048), Office of Water, Washington DC. DCN DW00899.
U.S. EPA, ASCE, and AWWA, 1996. Technology Transfer Handbook: Management of Water
Treatment Plant Residuals (EPA 625-R-95-008), Office of Research and Development,
Washington, DC. DCN DW03736.
7-15

SECTION 8
POLLUTANTS IN WATER TREATMENT PLANT RESIDUALS
This section identifies and discusses the pollutants present in WTP residuals
including the source of these pollutants. Section 9 presents pollutant loadings estimate for
discharges of these residuals and Section 10 discusses the environmental impacts of the
pollutants on discharge receiving streams.
EPA reviewed data sources to determine the presence of priority, conventional,
and nonconventional pollutant parameters in water treatment plant (WTP) residuals. EPA defines
priority pollutant parameters in Section 307(a)(1) of the Clean Water Act (CWA). Table 8-1 lists
the 126 specific priority pollutants listed in 40 CFR Part 423, Appendix A. For this industry
review, most of the priority pollutants listed in Table 8-1 were not identified as significant
contributors to WTP residuals. Section 304(a)(4) of the CWA defines conventional pollutant
parameters to include biochemical oxygen demand (BOD), total suspended solids (TSS), oil and
grease, pH, and fecal coliform bacteria. Nonconventional pollutant parameters are those that are
neither priority nor conventional pollutant parameters. This group includes nonconventional
metal pollutants, nonconventional organic pollutants, and other nonconventional pollutant
parameters such as chemical oxygen demand (COD).
EPA gathered data on pollutants from literature sources, including composition of
source water treatment chemicals (Cornwell, 2002) and pollutants identified by EPA’s Drinking
Water Program as a contaminant in finished drinking water (U.S. EPA, 2008), and discharge
monitoring reports (DMRs) from effluent discharges (U.S. EPA, 2007). Pollutants in WTP
residuals come from two sources: 1) treatment chemical addition (including by-product
formation); and 2) source water. The following subsections identify the pollutants commonly
found in residuals and wastewater discharged from WTPs.
8-1

Drinking Water Industry Report Section 8 – Pollutants in Water Treatment Plant Residuals
Table 8-1. Priority Pollutant List
a
1 Acenaphthene
2 Acrolein
3 Acrylonitrile
4 Benzene
5 Benzidine
6 Carbon tetrachloride
(tetrachloromethane)
7 Chlorobenzene
8 1,2,4-Trichlorobenzene
9 Hexachlorobenzene
10 1,2-Dichloroethane
11 1,1,1-Trichloroethane
12 Hexachloroethane
13 1,1-Dichloroethane
14 1,1,2-Trichloroethane
15 1,1,2,2-Tetrachloroethane
16 Chloroethane
17 Removed
18 Bis(2-chloroethyl) ether
19 2-Chloroethyl vinyl ether (mixed)
20 2-Chloronaphthalene
21 2,4,6-Trichlorophenol
22 Parachlorometa cresol (4-chloro-
3-methylphenol)
23 Chloroform (trichloromethane)
24 2-Chlorophenol
25 1,2-Dichlorobenzene
26 1,3-Dichlorobenzene
27 1,4-Dichlorobenzene
28 3,3'-Dichlorobenzidine
29 1,1-Dichloroethylene
30 1,2-Trans-Dichloroethylene
31 2,4-Dichlorophenol
32 1,2-Dichloropropane
33 1,3-Dichloropropylene (trans-1,3-
dichloropropene)
34 2,4-Dimethylphenol
35 2,4-Dinitrotoluene
36 2,6-Dinitrotoluene
37 1,2-Diphenylhydrazine
38 Ethylbenzene
39 Fluoranthene
40 4-Chlorophenyl phenyl ether
41 4-Bromophenyl phenyl ether
42 Bis(2-Chloroisopropyl) ether
43 Bis(2-Chloroethoxy) methane
44 Methylene chloride
(dichloromethane)
45 Methyl chloride (chloromethane)
46 Methyl bromide (bromomethane)
47 Bromoform (tribromomethane)
48 Dichlorobromomethane
(bromodichloromethane)
49 Removed
50 Removed
51 Chlorodibromomethane
(dibromochloromethane)
52 Hexachlorobutadiene
53 Hexachlorocyclopentadiene
54 Isophorone
55 Naphthalene
56 Nitrobenzene
57 2-Nitrophenol
58 4-Nitrophenol
59 2,4-Dinitrophenol
60 4,6-Dinitro-o-cresol (phenol, 2-
methyl-4,6-dinitro)
61 N-Nitrosodimethylamine
62 N-Nitrosodiphenylamine
63 N-Nitrosodi-n-propylamine (di-
npropylnitrosamine)
64 Pentachlorophenol
65 Phenol
66 Bis(2-ethylhexyl) phthalate
67 Butyl benzyl phthalate
68 Di-n-butyl phthalate
69 Di-n-octyl phthalate
70 Diethyl phthalate
71 Dimethyl phthalate
72 Benzo(a)anthracene (1,2-
benzanthracene)
73 Benzo(a)pyrene (3,4-
benzopyrene)
74 Benzo(b)fluoranthene (3,4-benzo
fluoranthene)
75 Benzo(k)fluoranthene (11,12-
benzofluoranthene)
76 Chrysene
77 Acenaphthylene
78 Anthracene
79 Benzo(ghi)perylene (1,12-
benzoperylene)
80 Fluorene
81 Phenanthrene
82 Dibenzo(a,h)anthracene (1,2,5,6-
dibenzanthracene)
83 Indeno(1,2,3-cd)pyrene (2,3-o-
phenylenepyrene)
84 Pyrene
85 Tetrachloroethylene
(tetrachloroethene)
86 Toluene
87 Trichloroethylene
(trichloroethene)
88 Vinyl chloride (chloroethylene)
89 Aldrin
90 Dieldrin
91 Chlordane (technical mixture &
metabolites)
92 4,4'-DDT (p,p'-DDT)
93 4,4'-DDE (p,p'-DDX)
94 4,4'-DDD (p,p'-TDE)
95 Alpha-endosulfan
96 Beta-endosulfan
97 Endosulfan sulfate
98 Endrin
99 Endrin aldehyde
100 Heptachlor
101 Heptachlor epoxide
102 Alpha-BHC
103 Beta-BHC
104 Gamma-BHC (lindane)
105 Delta-BHC
106 PCB-1242 (Arochlor 1242)
107 PCB-1254 (Arochlor 1254)
108 PCB-1221 (Arochlor 1221)
109 PCB-1232 (Arochlor 1232)
110 PCB-1248 (Arochlor 1248)
111 PCB-1260 (Arochlor 1260)
112 PCB-1016 (Arochlor 1016)
113 Toxaphene
114 Antimony (total)
115 Arsenic (total)
116 Asbestos (fibrous)
117 Beryllium (total)
118 Cadmium (total)
119 Chromium (total)
120 Copper (total)
121 Cyanide (total)
122 Lead (total)
123 Mercury (total)
124 Nickel (total)
125 Selenium (total)
126 Silver (total)
127 Thallium (total)
128 Zinc (total)
129 2,3,7,8-Tetrachloro-dibenzo-p-
dioxin (TCDD)
Source: 40 CFR Part 423, Appendix A.
a – Priority pollutants are numbered 1 through 129 but include 126 pollutants, because EPA removed three
pollutants (17, 49, and 50) from the list.
8-2

Drinking Water Industry Report Section 8 – Pollutants in Water Treatment Plant Residuals
8.1 OVERVIEW OF POLLUTANTS IN WATER TREATMENT PLANT
RESIDUALS
WTP residuals contain pollutants from the source water (concentrated when
removed from drinking water) and from treatment chemicals (including impurities and
disinfection by-products). Source water pollutants removed from potable drinking water include
solids, metals, and microorganisms. Pollutants from treatment chemical formulations include
active treatment chemical ingredients such as aluminum, calcium, and ammonia compounds, and
formulation impurities. Water treatment chemical impurities can concentrate into detectable
levels in residuals and recycle streams over time (Cornwell, 2002). Disinfection by-products
include bromate, chlorite, haloacetic acids, and trihalomethanes.
Common treatment chemicals listed in responses to the 2006 industry
questionnaire include the following (U.S. EPA, 2009):
• Chlorine (disinfection);
• Chlorine and ammonia (disinfection with chloramines);
• Conventional treatment:
— Aluminum chlorohydrate/polyaluminum chloride (PACl),
— Aluminum sulfate (alum),
— Iron-based coagulants (ferric chloride and ferric sulfide),
— Potassium permanganate,
— Polymer coagulants.
• Lime (precipitative) softening:
— Hydrated lime (Ca(OH)
2
),
— Caustic soda/sodium hydroxide (NaOH),
— Quick lime (CaO),
— Sodium carbonate/soda ash (Na
2
CO
3
).
• Powdered activated carbon;
• Granular activated carbon; and
• Fluoride.
Appendix B lists the compositions for some of the common treatment chemical
formulations.
8-3

Drinking Water Industry Report Section 8 – Pollutants in Water Treatment Plant Residuals
8.2 SOLIDS IN WATER TREATMENT PLANT RESIDUALS
Solids are the most common pollutant in WTP residuals. WTP residuals contain
both suspended and dissolved solids, which are also known as filterable and nonfilterable
residue. Suspended and dissolved solids concentrations are determined by filtering the solids
with a standard glass fiber filter and then drying them to a constant weight. The solids retained
on the filter are considered suspended solids, and the solids passing through the filter are
considered dissolved solids. Total solids are the sum of suspended and dissolved solids.
Suspended solids in WTP residuals include inorganic (e.g., silt, sand, clay, and
insoluble hydrated metal oxides) and organic matter (e.g., flocculated colloids and compounds
that contribute to color). Suspended solids may be measured using the parameters total
suspended solids or turbidity. Dissolved solids consist primarily of dissolved inorganic
compounds and can be found in ion exchange and membrane desalination concentrate waste
streams at high concentrations. One of the primary functions of WTPs is to remove solids from
the source water.
Solids in WTP residuals primarily come from the source water, but the addition of
treatment chemicals can add to the measured value (e.g., metals present in coagulants). Solids
from the source water may be concentrated in the residuals resulting in a higher solids
concentration than the source water solids concentration.
DMR data collected with the 2007 industry questionnaire includes TSS
concentrations for precipitative softening, conventional filtration (i.e., coagulation/filtration),
filtration only (includes microfiltration and ultrafiltration), membrane desalination, and ion
exchange plants. DMR data includes total dissolved solids (TDS) concentrations for ion
exchange plants. EPA included TSS and TDS in the pollutant loadings analysis (see Section 9).
A portion of the solids in WTP residuals are metals.
8-4

Drinking Water Industry Report Section 8 – Pollutants in Water Treatment Plant Residuals
8.3 PRIORITY AND NONCONVENTIONAL METALS IN WATER
TREATMENT PLANT RESIDUALS
A number of metals may be present in WTP residuals from the source water and
from source water treatment chemicals (and their impurities). Table 8-2 summarizes EPA’s
evaluation of the presence of priority metals and nonconventional metals in WTP residuals.
Metals, including iron, manganese, and mercury, listed in Table 8-2, may be present in source
water from natural erosion, land runoff, and industrial discharges. Aluminum salts, iron salts, and
polymers are commonly used as coagulants. Potassium permanganate is added to control taste
and odors, remove contaminants that cause color, control biological growth in treatment plants,
and remove iron and manganese. Lime products and caustic soda are added to reduce hardness.
Depending on the formulation, these treatment chemicals may contain metal impurities as listed
in Table 8-2.
WTPs remove metals from the source water to meet maximum contaminant levels
(MCLs) in the finished drinking water. The removed metals and metal constituents of treatment
chemicals become part of the residual waste streams. Permit writers select the appropriate
pollutants of concern when issuing discharge permits based on the pollutants in the source water
and type of treatment chemicals being added at the plant.
The following subsections discuss the active ingredient metals and other metals in
more detail.
8-5

Drinking Water Industry Report Section 8 – Pollutants in Water Treatment Plant Residuals
Table 8-2. Evaluation of Priority and Nonconventional Metals in Water Treatment Plant Residuals
Pollutant
Source Water
Contaminant
Removed from
Drinking Water
a
Present in Treatment Chemicals?
b
Aluminum-
Based
Coagulant
Iron-Based
Coagulant
Potassium
Permanganate
Organic
Polymers
Lime Products
Caustic Soda
Priority Metals
Antimony, total
Yes
No
Yes—Treatment
chemical impurity
No
No
No
No
Arsenic, total
Yes
No—Not present.
Beryllium, total
Yes
No—No data.
Cadmium, total
Yes
No
Yes—Treatment
chemical impurity
No
No
Yes—Treatment
chemical impurity
No
Chromium, total
Yes
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
No
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Copper, total
Yes
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
No
No
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Cyanide, total
Yes
No—No data.
Lead, total
Yes
No
Yes—Treatment
chemical impurity
No
No
No
No
Mercury, total
Yes
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
No
No
No
Nickel, total
No
e
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
No
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Selenium, total
Yes
No
No
Yes—Treatment
chemical impurity
No
No
No
Silver, total
Yes
No
No
Yes—Treatment
chemical impurity
No
No
No
Thallium
Yes
No—No data.
Zinc, total
Yes
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
8-6

Drinking Water Industry Report Section 8 – Pollutants in Water Treatment Plant Residuals
Table 8-2. Evaluation of Priority and Nonconventional Metals in Water Treatment Plant Residuals
Pollutant
Source Water
Contaminant
Removed from
Drinking Water
a
Present in Treatment Chemicals?
b
Aluminum-
Based
Coagulant
Iron-Based
Coagulant
Potassium
Permanganate
Organic
Polymers
Lime Products
Caustic Soda
Nonconventional Metals (Limited to those potentially in DWT Residuals)
Aluminum, total
Yes
Yes—Treatment
chemical addition
(active ingredient)
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Barium, total
Yes
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
No
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Calcium, total
c
No
f
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical addition
(active ingredient)
Yes—Treatment
chemical impurity
Cobalt, total
No
e
No
Yes—Treatment
chemical impurity
No
No
Yes—Treatment
chemical impurity
No
Fluoride, total
Yes
No—No data
Iron, total
Yes
Yes—Treatment
chemical impurity
Yes—Treatment
chemical addition
(active ingredient)
Yes—Treatment
chemical impurity
No
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Magnesium, total
No
f
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
No
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Manganese, total
Yes
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical addition
(active ingredient)
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Molybdenum
No
e
No
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
No
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Potassium, total
No
e
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical addition
(active ingredient)
No
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Silicon
No
e
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Sodium, total
d
No (but may be
present in source
water)
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical addition
(active ingredient)
8-7

Drinking Water Industry Report Section 8 – Pollutants in Water Treatment Plant Residuals
Table 8-2. Evaluation of Priority and Nonconventional Metals in Water Treatment Plant Residuals
Pollutant
Source Water
Contaminant
Removed from
Drinking Water
a
Present in Treatment Chemicals?
b
Aluminum-
Based
Coagulant
Iron-Based
Coagulant
Potassium
Permanganate
Organic
Polymers
Lime Products
Caustic Soda
Strontium, total
No
e
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
No
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Tin, total
No
e
No
Yes—Treatment
chemical impurity
No
No
No
No
Titanium, total
No
e
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Vanadium, total
Yes
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
No
No
Yes—Treatment
chemical impurity
No
Yttrium, total
No
e
No
No
Yes—Treatment
chemical impurity
No
Yes—Treatment
chemical impurity
No
Zirconium, total
No
e
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
Yes—Treatment
chemical impurity
No
Sources: U.S. EPA, 2008 and Cornwell, 2002.
a – Identified by EPA as a contaminant in drinking water.
b – “Yes” indicates that the metal was detected in at least one formulation sample. Specific formulation details are included in Appendix B.
c – Also an active ingredient in calcium hypochlorite (may be used for chlorination).
d – Also an active ingredient in sodium hypochlorite (may be used for chlorination).
e – Although not identified by EPA as a drinking water contaminant, metal may be present in certain source waters from natural materials (e.g., ores) or
industrial discharges.
f – Calcium and magnesium ions may be present in source water and removed via lime softening.
8-8

Drinking Water Industry Report Section 8 – Pollutants in Water Treatment Plant Residuals
8.3.1 Aluminum and Iron
As discussed in Section 6, WTPs use aluminum and iron salts as coagulants.
These metals are active ingredients in coagulants; and their use occurs at precipitative softening
and conventional filtration plants. These metals, along with coagulant impurities, become part of
the residual waste stream. In addition, the metals can be found in some source waters. Also,
DMR data collected with the 2007 industry questionnaire demonstrate the presence of aluminum
and iron in WTP discharges. As a result, EPA included aluminum and iron in the pollutant
loadings analysis (see Section 9).
8.3.2 Arsenic
Arsenic may be present at potentially high levels in the source water, especially
ground water sources. Sources of arsenic include natural sources (e.g., rocks, soil) and industrial
sources (e.g., use as a wood preservative). Higher concentrations of arsenic are typically found in
ground water compared to surface water. States in the western part of the United States tend to
have more public water systems with arsenic levels exceeding the MCL of 10 parts per billion
(ppb) for finished drinking water. Most of the systems in the Midwest and Northeast have arsenic
levels between 2 and 10 ppb (U.S. EPA, 2006a). Most systems with high levels of arsenic are
small systems (serving less than 10,000 people).
Of the systems affected by the OGWDW final arsenic rule (66 FR 6976,
January 22, 2001), 97 percent were small systems. EPA’s 2007 industry questionnaire focused
on large WTPs and the DMR data collected did demonstrated that arsenic was not present at
measurable concentrations. Therefore, EPA did not include arsenic in the pollutant loadings
analysis (see Section 9).
8.3.3 Calcium and Sodium
Calcium and sodium are active ingredients in lime products and caustic soda,
respectively. Lime products and caustic soda are added to reduce hardness (i.e., remove calcium
and magnesium from the source water). EPA has not set MCLs for these two pollutants in
drinking water. DMR data collected with the 2007 industry questionnaire demonstrated the
8-9

Drinking Water Industry Report Section 8 – Pollutants in Water Treatment Plant Residuals
presence of calcium, but no sodium. Therefore, EPA included only calcium in its pollutant
loadings analysis (see Section 9).
8.3.4 Fluoride
Fluoride occurs naturally in source water. WTPs may add fluoride to the drinking
water to promote healthy teeth; however, fluoride addition typically occurs at the end of the
source water treatment process. WTPs use finished drinking water to backwash filters; fluoride
may be present in residuals if added prior to finished water use as backwash. At the majority of
WTPs, the concentration of the fluoride in the wastewater is similar to the concentration in the
finished drinking water. DMR data collected with the 2007 industry questionnaire demonstrated
the presence of fluoride in discharges. EPA included fluoride in its pollutant loadings analysis
(see Section 9).
8.3.5 Manganese and Potassium
Manganese and potassium are active ingredients in potassium permanganate.
Potassium permanganate is added to control taste and odors, remove contaminants that cause
color, control biological growth in treatment plants, and remove iron and manganese from the
source water. EPA set secondary standards for manganese at 0.05 mg/L for drinking water. DMR
data collected with the 2007 industry questionnaire demonstrated the presence of manganese, but
not potassium. Therefore, EPA included only manganese in its pollutant loadings analysis (see
Section 9).
8.3.6 Additional Metals with DMR Data
As summarized in Table 8-2, metals may be present in the source water (and
concentrated in the WTP residuals) or in the treatment chemicals. The following metals are trace
contaminants in common WTP treatment chemicals and monitored by WTPs in the DMR data
collected by EPA:
• Barium;
• Cadmium;
8-10

Drinking Water Industry Report Section 8 – Pollutants in Water Treatment Plant Residuals
• Copper;
• Lead;
• Magnesium;
• Nickel; and
• Zinc.
EPA included these metals in its pollutant loadings analysis (see Section 9).
8.4 WTP POLLUTANTS FROM DISINFECTION
As discussed in Section 6.6, WTPs add disinfecting agents during source water
treatment to eliminate or inactivate microbiological populations. Primary disinfection occurs at
the front of the source water treatment process; disinfecting chemicals and any resulting by-
products might be found in WTP residuals generated later in the treatment process. Secondary
disinfection occurs at the clear well or in the distribution system to prevent microbiological
regrowth. If secondary disinfection occurs at the clear well, disinfecting chemicals and any
resulting by-products might be found in wastewaters where finished water is used for washing or
cleaning (e.g., filter backwash).
Chlorine is the most commonly used disinfectant. To disinfect with chlorine (or
chlorination), WTPs can use gaseous chlorine; calcium hypochlorite (Ca(OCl)
2
), an easily
dissolved solid containing 65 percent available chlorine; or sodium hypochlorite (NaOCl), a
solution with 5 to 15 percent chlorine. Other disinfectant chemicals include chloramines
(chlorine gas and ammonia), chlorine dioxide, and ozone. Most U.S. WTPs use gaseous chlorine
to disinfect drinking water (U.S. EPA, 2009).
Disinfection by-products (DBPs) form when disinfectants react with substances in
the source water, such as bromide and/or natural organic matter. EPA promulgated maximum
contaminant levels (MCLs) in drinking water for DBPs because they are potentially carcinogenic
(71 FR 478).
The DMR data collected with the 2007 industry questionnaire includes
concentrations for total residual chlorine and four DBPs: 1) bromodichloromethane, 2)
chloroform, 3) dibromochloromethane, and 4) trihalomethane. EPA did not have DMR data for
8-11

Drinking Water Industry Report Section 8 – Pollutants in Water Treatment Plant Residuals
chloramines discharges, but did have DMR data for ammonia concentrations in the effluent. EPA
included ammonia, total residual chlorine, the four DBPs listed above, and two additional DBPs,
bromoform and haloaectic acids, in the pollutant loadings analysis (see Section 9).
8.4.1 Chemistry of Chlorine Disinfection
Depending on the chemistry of the source water and wastewater, various forms of
chlorine and disinfection by-products may be present in WTP residuals. Figure 8-1 shows the
chemistry of how chlorine reacts when added during source water for disinfection (primary or
secondary) purposes (CDC, 2006; Block, 2000).
Chlorine Added
Total Residual Chlorine
The chlorine remaining
after the chlorine demand
Free Chlorine
Concentration of chlorine
available for disinfection
as HOCl or OCl-
Combined Chlorine
The concentration of
chlorine bound to nitrogen
compounds in the water
,
forming chloramines
Chlorine Demand
Reactions with organic
material
,
metals,
other
compounds present in
water prior to disinfection
Organic By-
Products
such as
:
Trihalomethanes
Haloacetic acids
Inorganic By-Products
such as
:
MnOCl
3
FeCl
3
Rapid
reaction
Slower reaction dependent
on the concentration of
free chlorine
Figure 8-1. Chemistry of Compounds Resulting from Chlorine Disinfection (CDC, 2006;
Block, 2000)
The chlorine chemistry shown above includes the following three components: 1)
chlorine added; 2) chlorine demand; and 3) total residual chlorine.
8-12

Drinking Water Industry Report Section 8 – Pollutants in Water Treatment Plant Residuals
8.4.1.1 Chlorine Added
As discussed above, chlorine may be added at several points during source water
treatment for disinfection. Primary disinfection is the addition of a disinfectant before
sedimentation or filtration to achieve desired inactivation of microorganisms. Secondary
disinfection is the addition of a disinfectant at the clear well and/or various points in the
distribution system to maintain a disinfectant residual in the finished water, preventing regrowth
of microorganisms.
8.4.1.2 Chlorine Demand
Chlorine demand is the chlorine consumed by inorganic and organic substances in
the water, not including amines. Chlorine reacts rapidly with inorganic substances, such as
metals (manganese and iron), hydrogen sulfide, and nitrites. Chlorine reacts more slowly with
organic substances, and the reaction depends on the amount of free chlorine available (U.S. EPA,
1999a). By-products formed during chlorination include inorganic chlorine compounds, such as
FeCl
3
and MnOCl
3
and organic chlorine compounds, such as trihalomethanes and haloacetic
acids.
8.4.1.3 Total Residual Chlorine
Total residual chlorine (TRC) is the amount of chlorine remaining after chlorine
demand. TRC includes combined chlorine and free chlorine. Combined chlorine is the chlorine
that has combined with amines to form chloramines. Although chloramines are a weaker
disinfectant than chlorine, some WTPs use them for secondary disinfection. To perform
disinfection with chloramines, WTPs inject chlorine, followed by ammonia into the distribution
main. Chloramines are more stable than free chlorine in distribution systems and therefore more
effective in controlling microorganism regrowth.
8-13

Drinking Water Industry Report Section 8 – Pollutants in Water Treatment Plant Residuals
There are three chloramine compounds, formed in the following conditions:
1. Monochloramine (NH
2
Cl):
NH
3
+ HOCl → NH
2
Cl + H
2
O;
pH near 6
2. Dichloramine (NHCl
2)
:
NH
2
Cl + HOCl → NHCl
2
+ H
2
O;
pH near 5
3. Nitrogen trichloride (NCl
3
) :
NHCl
2
+ HOCl → NCl
3
+ H
2
O;
Uncommon; undesirable
Free chlorine is the chlorine that is available for disinfection after other chlorine
compounds are formed, found as HOCl or OCl-, depending on pH.
8.4.2 Residual Disinfectants in Finished Drinking Water
Under the Safe Drinking Water Act (SDWA), EPA set requirements for drinking
water systems to ensure safe levels of disinfectants in the finished drinking water. The Total
Coliform Rule requires a minimum residual disinfectant level of 0.2 mg/L of total residual
chlorine for treated water entering the distribution system. Drinking water systems maintain
residual disinfectants in the finished water to ensure disinfection throughout the distribution
system.
EPA also set primary standards for the finished drinking water including the
maximum residual disinfectant levels (MRDLs) allowed. The MRDLs are:
• Chlorine: 4.0 mg/L;
• Chloramines (as chlorine): 4.0 mg/L; and
• Chlorine dioxide: 0.8 mg/L.
8.4.3 Disinfection By-Products
EPA identified four parameters in its DBP rules: chlorite, bromate, haloacetic
acids, and trihalomethanes (71 FR 478). EPA set standards for these because they are good
indicators of DBPs in disinfected drinking water, and because they are usually found at
measurable concentrations (71 FR 478). Haloacetic acids include monochloroacetic acid,
dichloroacetic acid, trichloroacetic acid, monobromoacetic acid, and dibromoacetic acid.
Trihalomethanes include chloroform (CHCl
2
, trichloromethane), bromodichloromethane
(CHCl
2
Br), bromoform (tribromomethane), and dibromochloromethane (CHClBr
2
).
8-14

Drinking Water Industry Report Section 8 – Pollutants in Water Treatment Plant Residuals
Chlorite is a by-product from disinfection with chlorine dioxide. Bromate is a by-
product from disinfection with ozone. Trihalomethanes and haloacetic acids by-products form
primarily from disinfection with chlorine, but also form when other disinfectants are used (71 FR
478).
WTPs can control DBPs by three methods: 1) removal of DBP precursors (i.e.,
natural organic matter), 2) modifying chlorination strategy, or 3) removing DBPs after
formation, where the last of these is the most difficult process. Most plants typically focus on
removing DBP precursors prior to chlorination. In general, aggregate DBP formation will
decrease as the removal of total organic carbons (TOCs) increases. Studies have found that
adding chlorine later (downstream) in the source water treatment process (e.g., adding after
sedimentation) results in a reduction of DBP formation. However, some plants use the addition
of chlorine to promote other pollutant removals prior to sedimentation (e.g., iron removal,
manganese removal, taste/odor control, and color removal). Plants may also decrease DBP
formation by the use of enhanced coagulation (U.S. EPA, 1999b).
21
8.5 PARAMETERS MEASURING ORGANIC MATTER AND OXYGEN IN
THE WATER IN WTP RESIDUALS
Plants can measure organic matter and oxygen content in the wastewater using
various parameters. Permit writers select which parameter works best for their NPDES
permitting program.
8.5.1 Biochemical Oxygen Demand
BOD is an estimate of the oxygen-consuming requirements of organic matter
decomposition under aerobic conditions. When WTP wastewaters are discharged to surface
waters, the microorganisms present in the naturally occurring microbial ecosystem decompose
21
“Enhanced coagulation” is the term used to define the process of improving removal of DBP precursors (natural
organic matter) by conventional filtration. “Enhanced softening” is the term used to define the process of improving
removal of DBP precursors by precipitative softening.
8-15

Drinking Water Industry Report Section 8 – Pollutants in Water Treatment Plant Residuals
the organic matter contained in the wastewater. The decomposition process consumes oxygen
and reduces the amount available for aquatic animals.
BOD determinations include estimates of the amount of oxygen required for the
degradation of both particulate and dissolved organic matter. Separation of these estimates is
accomplished by first filtering the sample to remove particulate organic matter and then
determining the BOD of the filtrate and dissolved BOD. The difference between BOD and
dissolved BOD (DBOD) is an estimate of the contribution of particulate matter to total BOD.
Also, BOD
5
typically measures carbonaceous oxygen demanding organic material in the
wastewater (CBOD). Nitrogenous oxygen demanding material (NBOD or NOD) is not likely to
be a major concern for WTP wastewaters, as it is for certain nitrogen-containing industrial and
municipal wastewaters and associated treatment systems.
DMR data collected with the 2007 industry questionnaire includes concentration
of BOD in effluent discharges from conventional filtration plants. The data also includes CBOD
concentrations in effluent discharges from membrane desalination and ion exchange plants. EPA
included BOD and CBOD in the pollutant loadings estimate (see Section 9).
8.5.2 Dissolved Oxygen
Dissolved oxygen (DO) measures the amount of oxygen in the water. Water
bodies both produce and consume oxygen. A water body gains oxygen from the atmosphere and
from plants as a result of photosynthesis. Running water, because of its churning, dissolves more
oxygen than still water. Respiration by aquatic animals, decomposition, and various chemical
reactions consume oxygen.
WTP residuals may contain organic materials that are decomposed by
microorganisms, using oxygen in the process. The amount of oxygen used is measured as BOD
(discussed above). If more oxygen is consumed than produced, DO levels decline and some
sensitive animals may move away, weaken, or die.
8-16

Drinking Water Industry Report Section 8 – Pollutants in Water Treatment Plant Residuals
DO levels fluctuate seasonally and over a 24-hour period. The level also varies
with water temperature and altitude. Cold water holds more oxygen than warm water, and water
holds less oxygen at higher altitudes.
EPA received DO data with the DMR data collected with the 2007 industry
questionnaire. Because DO requirements are to maintain a minimum level, EPA did not include
this pollutant in the pollutant loadings estimate (see Section 9).
8.6 OTHER POLLUTANTS IN WTP
Other pollutant parameters found in residuals are primarily contaminants removed
from the source water to produce finished drinking water. The pollutants discussed in this section
include chloride, nitrogen, pH, phosphorous, and radionuclides.
8.6.1 Chloride
Chloride (Cl-) is a common anion in wastewaters and natural waters. Excessively
high chloride concentrations in surface waters can impair their use as source waters for potable
water supplies. If sodium is the predominant cation present, the water will have an unpleasant
taste due to the corrosive action of chloride ions. Chloride is a constituent of TDS; dissolved
solids are removed using membrane desalination and ion exchange processes. DMR data
collected with the 2007 industry questionnaire includes concentrations of chlorides in effluent
discharges from membrane desalination and ion exchange plants. EPA used these concentrations
in its pollutant loadings analysis (see Section 9).
8.6.2 Nitrogen
Nitrogen may be present in WTP residuals (removed from the source water).
WTPs are required to meet primary drinking water standards for nitrate (measured as nitrogen)
and nitrite (measured as nitrogen). There are several parameters to measure forms of nitrogen,
including total nitrogen, total Kjeldahl nitrogen (TKN), ammonia nitrogen (NH4-N), and nitrite
plus nitrate nitrogen (NO2 + NO3-N). TKN is defined as the sum of organic nitrogen and free
ammonia. DMR data collected with the 2007 industry questionnaire includes total nitrogen
8-17

Drinking Water Industry Report Section 8 – Pollutants in Water Treatment Plant Residuals
concentrations and ammonia concentrations. EPA used these concentrations in its pollutant
loadings analysis (see Section 9).
8.6.3 pH
WTPs adjust the pH to optimize source water treatment, and the addition of lime
for softening raises the pH of the water. The hydrogen-ion concentration in an aqueous solution
is represented by the pH, which is defined as the negative logarithm of the hydrogen-ion
concentration in a solution. On the pH scale ranging from zero to 14, a value of seven represents
neutral conditions—the concentrations of hydrogen (H+) and hydroxyl ions (OH-) are equal. pH
values less than seven indicate acidic conditions and values greater than seven represent basic
conditions.
EPA received pH data with the DMR data collected with the 2007 industry
questionnaire. Because pH cannot be expressed as pounds in the discharge, EPA did not include
this pollutant in the pollutant loadings estimate (see Section 9).
8.6.4 Phosphorus
The sources of phosphorus in WTP residuals and wastewater discharges include
the source water and treatment chemicals for scale and corrosion control. In marine waters,
phosphorus is not as much of a concern because of relatively high naturally occurring
phosphorus concentrations. The impact of phosphorus in wastewater discharges into estuaries
varies—in general, impacts decrease as salinity levels increase. DMR data collected with the
2007 industry questionnaire includes phosphorus concentrations in effluent discharges. EPA
used these concentrations in its pollutant loadings analysis (see Section 9).
8.6.5 Radionuclides
Low levels of radioactive contaminants, or radionuclides, occur in most drinking
water sources and do not pose a public health risk. However, some drinking water sources have
elevated radionuclide levels, usually occurring naturally (from certain rock types). Radionuclides
regulated by EPA include the following:
8-18

Drinking Water Industry Report Section 8 – Pollutants in Water Treatment Plant Residuals
• Combined radium -226/-228: occurs naturally in some drinking water
sources.
• (Adjusted) Gross alpha: occurs naturally in some drinking water sources.
• Beta particle and photon radioactivity: contamination from facilities using
or producing radioactive materials.
• Uranium: occurs naturally in some drinking water sources.
Some drinking water sources located in the Midwest have elevated levels of
radium -226/-228, while some sources in the West have elevated uranium levels (U.S. EPA,
2006c). DMR data collected with the 2007 industry questionnaire includes concentration for
radionuclides at some WTPs. However, since the presence of radionuclides is dependent on the
source water, EPA did not use the DMR data to estimate pollutant loadings for the industry (see
Section 9).
8.7 REFERENCES
American Public Health Association (APHA), 1995. Standard Methods for the Examination of
Water and Wastewater, 19
th
edition, Washington, DC.
Cornwell, 2002. David A. Cornwell, Michael J. Macphee and Richard Brown Trace
Contaminants in Drinking Water Chemicals, American Water Works Association (AWWA)
Research Foundation. Document Control Number (DCN) DW03737.
Block, 2000. Disinfection, Sterilization, and Preservation. Block, Seymour S. Stanton.
Lippincott (ISBN 0683307401). 2000. DCN DW03744.
Center for Disease Control (CDC), 2006. Chlorine Residual Testing Fact Sheet, CDC SWS
Project. Retrieved from http://cdc.gov/safewater/publications_pages/pubs_factsheets.htm. May
23, 2006. DCN DW03745.
U.S. Environmental Protection Agency (EPA), 1983. Methods for Chemical Analysis of Water
and Wastes (EPA 600-4-79-020), Office of Research and Development, Washington, DC.
U.S. EPA, 1999a. Disinfection Profiling and Benchmarking Guidance Manual. EPA 815-R-99-
013. August 1999. DCN DW03743.
U.S. EPA, 1999b. Alternative Disinfectants and Oxidants Guidance Manual (EPA 815-R-99-
014), Office of Water, Washington, DC. DCN DW00647.
8-19

Drinking Water Industry Report Section 8 – Pollutants in Water Treatment Plant Residuals
U.S. EPA, 2006a. Arsenic in Drinking Water (website), Office of Water. Retrieved from
http://www.epa.gov/safewater/arsenic/basicinformation.html, last updated February 28, 2006.
DCN DW01070.
U.S. EPA, 2006c. Radionuclides in Drinking Water (website), Office of Water. Retrieved from
http://www.epa.gov/safewater/standard/pp/radnucpp.html, last updated February 28, 2006. DCN
DW00943.
U.S. EPA, 2007. Phase I Discharge Monitoring Report (DMR) Database, Office of Water,
Washington, DC. DCN DW03703.
U.S. EPA, 2008. National Primary Drinking Water Standards (List of Drinking Water
Contaminants and MCLs) (original document published in 2003, DCN DW00657), Office of
Ground Water and Drinking Water, Washington, DC. Retrieved from
http://www.epa.gov/safewater/contaminants/index.html Accessed April 2008 for updates.
U.S. EPA, 2009. Drinking Water Survey Response Database – Technical Data for the 2006
Drinking Water Industry Questionnaire. Office of Water, Washington, DC. DCN DW03790.
8-20

SECTION 9
WATER TREATMENT PLANT POLLUTANT DISCHARGE
ESTIMATES
As part of its effluent guidelines review process, EPA developed a variety of tools
and methodologies to evaluate effluent discharges from various industrial categories. One of the
main tools EPA used is an estimate of pollutant loadings being discharged from facilities within
an industry sector. This section discusses how EPA estimated pollutant loadings for the drinking
water treatment (DWT) industry.
Pollutant loadings are the estimated amount of pollutants in water treatment plant
(WTP) residuals currently being discharged to surface waters, whether directly from the plant or
indirectly from publicly owned treatment works (POTWs) after taking POTW treatment
effectiveness into account (i.e., pollutants that pass through the POTW). As part of the drinking
water industry review, EPA estimated pollutant loadings from water treatment plants (WTPs) in
the U.S. that serve more than 10,000 people. These loadings include contaminants in the source
water that are removed to produce drinking water, and ingredients present in treatment chemicals
added by the WTP. EPA did not have data to quantify the pollutant discharges attributed to
source water contaminants and those attributed to treatment chemical addition.
EPA estimated discharges for bulk parameters and chemical-specific parameters.
Bulk parameters for the DWT pollutant loadings analysis include biochemical oxygen demand
(BOD), carbonaceous BOD (CBOD), total nitrogen, total dissolved solids (TDS) and total
suspended solids. The pollutant loadings estimated for bulk parameters may include the
chemical-specific pollutant loading (e.g., TSS loadings include metals such as aluminum
loadings). Because some portion of the chemical-specific pollutants are included in the bulk
pollutant estimates, the two estimates should never be summed as this would constitute double
counting of pollutants and result in an overestimate of the total pollutant loadings from DWT.
EPA presents both bulk and chemical-specific parameters in this report since they offer different
types of information but these estimates will always be presented separately to emphasize the
non-additive nature of the data.
9-1

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Overall, EPA estimated that the discharges from the industry are 574 million
pounds of bulk parameters per year and 352 million pounds per year of chemical-specific
parameters, including metals and pollutants from disinfection with chlorine (chlorination). The
majority of the bulk parameter loadings (over 98 percent) are TSS: 314 million pounds per year,
primarily from precipitative softening plants, and TDS: 252 million pounds per year, primarily
from ion exchange/adsorption plants. In EPA’s loadings analysis most of the chemical-specific
parameter releases (over 98 percent) are due to the following five pollutants:
1. Chlorides: 326 million pounds per year from membrane desalination and
ion exchange/adsorption plants;
2. Calcium: 14.4 million pounds per year from precipitative softening and
coagulation/filtration plants;
3. Magnesium: 4.2 million pounds per year from precipitative softening and
coagulation/filtration plants;
4. Lead: 1.97 million pounds per year, primarily from coagulation/filtration
plants; and
5. Aluminum: 1.48 million pounds per year, primarily from
coagulation/filtration plants.
In addition to the pounds per year, EPA also estimated the toxic-weighted pound
equivalent (TWPE) for the loadings parameters to determine the relative toxicity of DWT
discharges
22
. EPA used toxic weighting factors (TWFs) that are specific to each chemical. EPA
estimated 415,000 toxic-weighted pounds per year. Most of the TWPE (85 percent) is due to five
pollutants:
1. Total Residual Chlorine: 120,000 pound equivalents per year (lb-eq/yr);
2. Aluminum: 88,600 lb-eq/yr;
3. Copper: 60,700 lb-eq/yr;
4. Manganese: 41,800 lb-eq/yr; and
22
The DWT discharges include both the source water contaminants removed to produce drinking water and
ingredients in treatment chemicals added by the WTP. EPA does not have sufficient source water characteristic data
to determine the proportion of the total discharge loadings that come from source water contaminates versus
material added by the WTP facilities as part of the drinking water treatment process.
9-2

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
5. Fluoride: 41,100 lb-eq/yr.
This section describes EPA’s pollutant loadings analysis in the following
subsections:
• Section 9.1: Data sources used for the pollutant loadings analysis;
• Section 9.2: Methodology to estimate pollutant loadings using model
plants;
• Section 9.3: Selection of pollutants to include in the loadings estimates;
• Section 9.4: Development of long-term averages for pollutants;
• Section 9.5: Pollutant loadings estimate for model plants; and
• Section 9.6: National discharge estimate of pollutants from WTPs serving
more than 10,000 people.
9.1 DATA SOURCES FOR THE POLLUTANT LOADINGS ANALYSIS
For this analysis, EPA estimated pollutant loadings discharged in the base year of
the questionnaire (2006). EPA used the following data sources as part of the pollutant loadings
analysis:
• Discharge monitoring report (DMR) data from WTPs (U.S. EPA,
2007): These data were used to calculate average pollutant concentrations
in the discharges for model plants by source water treatment type and type
of residuals treatment. EPA also used the flow rates reported to calculate
average direct discharge flow rates for model plants by source water
treatment type. EPA used data from 108 WTPs (direct dischargers with
completed survey responses and submitted DMR data). EPA
supplemented pollutant concentration data for pollutants resulting from
chlorination using four additional WTPs with DMR data.
• 2006 WTP Questionnaire Response Database – Technical Data (U.S.
EPA, 2009a): EPA used the survey responses to classify WTPs with DMR
data into the four characteristics used to define model plants (see below).
EPA also used the flow rates for indirectly-discharging plants to calculate
average indirect discharge flow rates for model plants by source water
treatment type.
9-3

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
• EPA published maximum contaminant levels (MCLs) (U.S. EPA,
2008b): MCLs are the maximum amount of a source water contaminant
allowed in the finished drinking water. For one pollutant without DMR
data (haloacetic acids), EPA used the MCL to estimate the average
concentration discharged by model plants.
• EPA National Estimates: WTP Counts for Pollutant Loadings (see
Appendix A) EPA used the survey responses to classify all WTPs in the
sample frame into the four characteristics used to define model plants (see
below).
9.2 METHODOLOGY TO ESTIMATE POLLUTANT LOADINGS USING
MODEL PLANTS
EPA used a model plant approach to estimate pollutant loadings from the drinking
water treatment industry. EPA estimated the pollutant loadings being discharged from each of
the model plants and then calculated national discharges by multiplying the model treatment
plant loadings by the number of WTPs represented by that model plant. A WTP may represent
multiple types of source water treatment, but was counted only one time in the totals.
9.2.1 Model Plant Development
EPA used four factors representing the different types of WTPs in the U.S. to
develop the model plants. EPA selected these four major factors because they govern the amount
of pollutants discharged in residuals. The four factors are:
• Type of WTP (such as coagulation and filtration or precipitative
softening);
• Type of residuals treatment in place;
• WTP size; and
• Discharge status (i.e., direct or indirect).
Applying these four factors led to the development of distinct model plants. Each of the factors is
described in detail below.
9-4

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
9.2.1.1 Type of WTP
Based on data collected for the industry review, EPA determined that pollutant
concentrations in residuals would vary by source water treatment type and type of residuals
treatment in place. The five source water treatment types that EPA included in its analysis are the
following:
• Precipitative (i.e., lime) softening: includes all plants performing
precipitative softening.
• Coagulation & filtration: includes conventional filtration plants, direct
filtration plants, microfiltration (MF) plants also performing coagulation;
and ultrafiltration (UF) plants also performing coagulation.
• Filtration only: includes plants performing filtration, MF, and UF without
coagulation.
• Membrane desalination: includes reverse osmosis (RO), nanofiltration
(NF), electrodialysis (ED), and electrodialysis reversal (EDR) plants.
• Ion exchange & adsorption: includes plants performing ion exchange or
adsorption (e.g., granular activated carbon).
9.2.1.2 Type of Residuals Treatment
EPA identified two groups of residuals treatment that would affect the pollutant
concentration in the effluent: 1) solid/water separation and 2) dechlorination. For most
pollutants, WTPs use solid/water separation to treat residuals. For pollutants resulting from
disinfection with chlorine, WTPs use dechlorination to treat the residuals. EPA determined that
pollutant concentrations in residuals would vary by the type of residuals treatment in place. For
pollutants other than those from disinfection with chlorine, EPA used two residuals treatment
types for model plants: 1) solid/water separation or 2) no solid/water separation. For pollutants
resulting from disinfection with chlorine, EPA also used two residuals treatment types for model
plants: 1) dechlorination; and 2) no dechlorination.
9-5

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
9.2.1.3 WTP Size/Flow Rate
In addition to the two characteristics affecting pollutant concentrations in
discharges (source water treatment and residuals treatment), pollutant loadings are based on the
volume of wastewater residuals generated. EPA determined that the discharge flow rate would
vary by source water treatment type, plant size (correlated to population served), and discharge
status (direct or indirect). EPA used the following population served size categories for the
model plants:
• Population served between 10,001 and 50,000 people;
• Population served between 50,001 and 100,000 people;
• Population served between 100,001 and 500,000 people; and
• Population served greater than 500,000 people.
9.2.1.4 Discharge Status
In addition to using the discharge status (direct or indirect) to determine model
plant effluent flow rates, EPA also used the discharge status when calculating pollutant loadings.
For model plants discharging indirectly (i.e., wastewater treated by a POTW prior to discharge in
waters of the U.S.), EPA accounted for the pollutants removed by the POTW (i.e., loadings are
for pollutants that pass through the POTW).
9.2.2 Estimation of Model Plant Pollutant Loadings
EPA estimated pollutant loadings for each model plant and pollutant parameter
for the base year of 2006 using the equations below:
Model Plant Load = (Concentration × Flow × Conversion Factor) (Eq. 9-1)
where:
Model Plant Load = Pollutant loadings, in pounds per year (lbs/year).
Concentration = Annual average pollutant concentration, in milligrams per
liter (mg/L).
Flow = Production-based discharge flow rate, in million gallons per
day.
Conversion factor = 8.345 (to convert the loadings into lbs/year; derived from
3.784 L/gal × 2.2 lbs/kg) x 365 days per year.
9-6

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
EPA estimates the pollutant loadings from indirect dischargers to account for
pollutant discharges that pass through the POTW to surface waters. Indirect discharges are
treated at POTWs prior to discharge and EPA takes that treatment into account when calculating
pollutant loadings. For indirect dischargers, EPA uses the results from Equation 9-1 and accounts
for treatment at the POTW prior to discharge to surface waters using Equation 9-2:
Load
POTW
= (1 – POTW % Removal) × Model Plant Load (Eq. 9-2)
where:
Load
POTW
= Pollutant loadings discharged to surface water after
treatment at the POTW, in pounds per year (lb/year).
Model Plant Load = Pollutant loadings discharged to the POTW from Equation
9-1 for each indirect discharger, in pounds per year
(lb/year).
POTW % Removal = Percent removal at the POTW, shown in Appendix C
Most of the POTW percent removal values are based on data from the Fate of
Priority Pollutants in Publicly Owned Treatment Works and National Risk Management
Research Laboratory (NRMRL) Treatability Database (U.S. EPA, 1982 and U.S. EPA, 1994)
and are presented in Appendix C. The pollutant loadings and associated removals for indirect
dischargers presented in this report represent pass through discharge from POTWs to receiving
streams using the above equation.
EPA also estimated toxic-weighted pound equivalent (TWPE) pollutant loadings.
To calculate TWPE, EPA multiplied the annual load (lb/yr) by a toxic weighting factor (TWF).
TWFs account for differences in toxicity across pollutants and provide the means to compare
mass loadings of different pollutants on the basis of their toxic potential. EPA multiplies a mass
loading of a pollutant in pounds per year (lb/yr) by a pollutant-specific weighting factor to derive
a "toxic-equivalent" loading (lb-equivalent/yr), or TWPE. EPA has developed TWFs for more
than 1,900 pollutants based on aquatic life and human health toxicity data, as well as
physical/chemical property data. EPA calculated TWPE using Equation 9-3. TWPEs do not
apply to conventional pollutants or bulk parameters.
TWPE (lb-eq-yr) = Annual Load (lb/yr) × TWF (Eq. 9-3)
9-7

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
The TWFs used for the pollutant loading estimates are presented in Appendix D
(U.S. EPA, 2006).
9.3 MODEL PLANT CONCENTRATION ESTIMATION
To estimate model plant loadings, Equation 9-1 lists two variables: concentration
and flow rate. This section discusses how EPA estimated pollutant concentrations in the model
plant effluent discharges and Section 9.4 discusses how EPA estimated effluent discharge flow
rates for the model plants.
9.3.1 Selection of Pollutant Parameters for Pollutant Loadings Analysis
EPA identified two groups of pollutant parameters for the loadings analysis based
on type of residual treatment that affects the pollutant discharges: 1) pollutants resulting from the
disinfection with chlorine (chlorination); and 2) all other pollutants. Chlorination pollutants
include: total trihalomethanes, chloroform, bromodichloromethane, bromoform,
dibromochloromethane, haloacetic acids, chloramines, and total residuals chlorine. To treat
chemicals resulting from disinfection with chlorine, WTPs perform dechlorination. To treat all
other pollutants, WTPs perform solid/water separation.
EPA selected a subset of pollutants for the model plant loadings estimates based
on three main factors: 1) ability to estimate pollutant loadings in pounds per year (lbs/yr), 2)
availability of concentration data from the DMR submittals, and 3) presence of the pollutant in
the residuals for the source water treatment type.
23
EPA included three pollutants without DMR data in the pollutant loadings
analysis. Bromoform, haloacetic acids, and chloramines are by-products of disinfection with
chlorine. EPA estimated loadings for bromoform and haloacetic acids using a mass balance
approach, which allows the estimation of pollutant loadings without DMR data. For bromoform,
23
The memorandum entitled Pollutant Loadings Estimates for Drinking Water Treatment Plants: Model Plants and
National Estimates (ERG, 2009) details the selection of pollutants for the loadings analysis.
9-8

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
EPA transferred the effluent concentration from chloroform. For haloacetic acids, EPA used the
drinking water MCL to estimate pollutant loadings. For chloramines, EPA performed a
qualitative review of the discharges of chloramines from WTPs.
EPA selected 27 pollutants to include in the loadings analysis. For pollutants
resulting from chlorination, EPA estimated discharge concentrations based on two factors: 1)
whether the WTP disinfects with chlorine and 2) whether residuals are treated using
dechlorination prior to discharge). If the WTP does not use chlorine for disinfection, EPA set the
loadings for the chlorination pollutants equal to zero. EPA assumed that WTPs that do not
disinfect with chlorine would not discharge pollutants resulting from chlorination. EPA did not
differentiate between source water treatment types (e.g., assumed concentrations would be the
same for precipitative softening plants as for plants with only filtration). Table 9-1 presents the
pollutants selected for the loadings estimates for each source water treatment type.
Table 9-1. Pollutants Included in the Loadings Estimates
Parameter
Precipitative
Softening
Coagulation
and Filtration
Filtration
Only
Membrane
Desalination
Ion Exchange
and Adsorption
Conventionals
BOD
X
a
X
X
a
CBOD5
X
X
TSS
X
X
X
X
X
Other Solids
TDS
c
X
Chlorides
X
X
Nitrogen
Nitrogen, Total
c
X
a
X
X
a
X
X
Ammonia
X
a
X
X
a
X
X
Metals
Aluminum
X
X
X
Barium
X
a
X
Cadmium
X
X
Calcium
X
a
X
Copper
X
a
X
X
X
Fluoride
X
X
X
X
X
Iron
X
X
X
X
X
Lead
X
a
X
X
a
X
Magnesium
X
a
X
Manganese
X
X
X
X
9-9

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-1. Pollutants Included in the Loadings Estimates
Parameter
Precipitative
Softening
Coagulation
and Filtration
Filtration
Only
Membrane
Desalination
Ion Exchange
and Adsorption
Nickel
X
a
X
Phosphorus
X
a
X
X
a
X
X
Zinc
X
a
X
X
Pollutants from Chlorination and Disinfection By-Products
Bromodichloromethane
X
b
Bromoform
X
b
Chlorine, Total Residual
X
b
Chloroform
X
b
Dibromochloromethane
X
b
Haloacetic acids
X
b
Trihalomethane
X
b
a – Transfer concentrations from coagulation/filtration source water treatment type. Note that all but three of the
survey respondents that perform precipitative softening also perform coagulation and filtration (U.S. EPA, 2009a);
therefore EPA included the same pollutants in the loadings analysis for each model plant type. For filtration only,
EPA transferred pollutant concentrations from coagulation and filtration for source water contaminant pollutants that
may be concentrated in the residuals.
b – Pollutant discharges expected only from WTPs that disinfect with chlorine. For pollutant loading estimates, EPA
did not group chlorination chemical concentrations by source water treatment type.
c – Bulk parameters represent more than one pollutant. For example, Total Nitrogen includes ammonia nitrogen
(NH
3
) as well as organic nitrogen, nitrate, and nitrite. EPA estimated loads for total nitrogen in lbs/yr. EPA
estimated loads for ammonia nitrogen in lbs/yr and TWPE/yr. EPA does not estimate TWPE for bulk parameters,
because TWFs apply to specific chemicals. TDS is also a bulk parameter that includes chlorides.
In addition to grouping DMR data by source water treatment type, EPA used the
survey response database (U.S. EPA, 2009a) to determine whether the WTP performed residuals
treatment prior to discharge. WTPs use solid/water separation to remove most pollutants from
the residuals. Three of the plants fell under two source water treatment types. EPA used the
plant’s DMR data to characterize discharges from both source water treatment types. Table 9-2
summarizes the WTPs with DMR data and whether solid separation is used to treat residuals.
9-10

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-2. Type of Source Water Treatment and Residuals in Place (Solid/Water
Separation) for WTPs with DMR Data
Source Water Treatment Type
Total Number of
Plants
Number of Plants without
Solid/Water Separation
(Untreated)
Number of Plants with
Solid/Water Separation
(Treated)
Precipitative softening
24
6
18
Coagulation/filtration
76
6
70
Filtration only (including MF and
UF)
5
1
4
Membrane desalination
a
2
2
0
Ion exchange and adsorption
b
4
1
3
Total
c
108
15
93
Source: U.S. EPA, 2009a and U.S. EPA, 2007.
a – DMR data available for high pressure membrane (reverse osmosis and nanofiltration) plants. Data were not
available for electrodialysis and electrodialysis reverse plants; assume discharge similar pollutants and at similar
concentrations to high pressure membrane plants. Desalination membrane plants typically do not treat the
concentrate prior to discharge (Malmrose, et al., 2004).
b – DMR data available for ion exchange plants. Data were not available for adsorption plants; assume discharge
similar pollutants and at similar concentrations to ion exchange plants.
c – A WTP may represent multiple types of source water treatment, but was counted only one time in the totals.
To remove pollutants resulting from disinfection with chlorine, WTPs use
dechlorination to treat residuals. Table 9-3 summarizes the WTPs with DMR data including
whether the WTP uses chlorine for disinfection and whether dechlorination is used to treat
residuals.
9-11

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-3. Type of Source Water Treatment and Residuals in Place (Dechlorination) for
WTPs with DMR Data
Source Water
Treatment Type
Total
Number of
Plants
Number of Plants
Performing
Chlorination
Number of Plants
without Dechlorination
(Untreated)
Number of Plants
with Dechlorination
(Treated)
Lime softening
24
22
17
5
Coagulation/filtration
76
69
47
22
Filtration only (including
MF and UF)
5
4
3
1
Membrane desalination
a
2
2
1
1
Ion exchange and
adsorption
b
4
4
2
2
Total
c
108
d
98
68
30
Source: U.S. EPA, 2009a and U.S. EPA, 2007.
a – DMR data available for high pressure membrane (reverse osmosis and nanofiltration) plants. Data were not
available for electrodialysis and electrodialysis reverse plants; assume discharge similar pollutants and at similar
concentrations to high pressure membrane plants.
b – DMR data available for ion exchange plants. Data were not available for adsorption plants; assume discharge
similar pollutants and at similar concentrations to ion exchange plants.
c – A WTP may represent multiple types of source water treatment, but was counted only one time in the totals.
d – EPA used DMR data collected with the 2007 industry questionnaire from an additional four WTPs to
characterize discharge of disinfection by-products (U.S. EPA, 2007). These four WTPs are not included in the above
total because a complete survey review was not completed for the four WTPs; therefore, these four WTPs are not
included in the technical survey response database (U.S. EPA, 2009a).
9.3.2 Development of Long-Term Average Concentrations for Pollutants
EPA estimated the annual average pollutant concentrations (long-term averages)
for each model plant, based on source water treatment type and residuals treatment in place. EPA
does not expect WTP size or discharge status to affect the concentration in the effluent discharge.
EPA used DMR data from WTPs in the survey database with matching source water treatment
type and residuals treatment in place to estimate long-term average concentrations. EPA used
alternate approaches for two pollutants without DMR data:
1. Bromoform (tribromomethane): Transfer from similar trihalomethane
(chloroform); and
2. Haloacetic acids (5HAA’s): Use MCL as concentration.
EPA used the DMR data supplied with the 2007 industry questionnaire response
to estimate annual averages for each WTP and pollutant. To calculate the annual average
pollutant concentration, EPA took the arithmetic mean of the samples taken in 2006. For samples
9-12

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
showing presence of a chemical but at concentrations below detection limits, EPA used one-half
of the method detection limit value to estimate pollutant loadings. For chemicals never detected
in the effluent, EPA used a concentration of zero for the loadings estimates.
EPA averaged the DMR pollutant concentrations for each source water treatment
type and residuals treatment in place (i.e., model plant). For most pollutants, EPA calculated
annual average pollutant concentrations by the source water treatment type and whether the WTP
treated residuals using solid/water separation. For pollutants resulting from disinfection with
chlorine, EPA differentiated the average pollutant concentration only by whether or not the plant
used chlorine for disinfection and performed dechlorination. EPA describes the long-term
average calculations in more detail in the memorandum entitled, Pollutant Loadings Estimates
for Drinking Water Treatment Plants (ERG, 2009).
Table 9-4 presents the model plant long-term average concentrations for all
pollutants except those resulting from chlorination. These are grouped by source water treatment
type and residuals treatment in place. EPA did not apply any toxic weighting factors (TWFs) to
the long-term averages; TWFs are applied to the pounds per year loadings.
Table 9-5 presents the long-term average concentrations for pollutants resulting
from chlorination. These are grouped only by the presence of dechlorination as part of residuals
treatment. EPA did not apply any toxic weighting factors (TWFs) to the long-term averages;
TWFs are applied to the pounds per year loadings.
9-13

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-4. Long-Term Average Concentrations from DMR Data by Source Water Treatment Type and Residuals Treatment
(mg/L)
Pollutant
Precipitative Softening
Coagulation and
Filtration
Filtration only
Membrane Desalination
Ion Exchange and
Adsorption
Solids
Separation
No Solids
Separation
Solids
Separation
No Solids
Separation
Solids
Separation
No Solids
Separation
Solids
Separation
No Solids
Separation
Solids
Separation
No Solids
Separation
Conventionals
BOD
1.44 (b)
1.44 (b)
1.44
1.44 (e)
1.44 (b)
1.44 (b)
(d)
(d)
(d)
(d)
CBOD5
(d)
(d)
(d)
(d)
(d)
(d)
1.00 (f)
1.00
1.00 (f)
1.00
TSS
5.89
1,430
54.5
135
2.62
22.5
2.86 (f)
2.86
6.38 (a)
Other Solids
TDS
(d)
(d)
(d)
(d)
(d)
(d)
(d)
(d)
8,570
8,570 (e)
Chlorides
(c)
(c)
(c)
(c)
(c)
(c)
7,120 (f)
7,120
2,930
7,120
Nitrogen
Nitrogen, Total
3.64 (b)
3.64
3.64
3.64 (e)
3.64 (b)
3.64 (b)
2.95 (f)
2.95
0.472
0.908
Ammonia
0.482 (b)
0.482 (b)
0.482
0.482 (e)
0.482 (b)
0.482 (b)
1.55 (f)
1.55
0.0894
0.0894 (e)
Metals
Aluminum
0.177 (a)
2.16 (a)
0.919
0.919 (e)
(d)
(d)
(d)
(d)
Barium
0.0100 (b)
0.0100 (b)
0.0100
0.0100 (e)
(d)
(d)
(d)
(d)
(d)
(d)
Cadmium
(d)
(d)
(d)
(d)
(d)
(d)
0.00104 (f)
0.00104
0.00104 (f)
0.00104
Calcium
8.73 (b)
8.73 (b)
8.73
8.73 (e)
(d)
(d)
(d)
(d)
(d)
(d)
Copper (g)
0.0693 (b)
0.0693 (b)
0.0693
0.0693 (e)
(d)
(d)
0.000891(f)
0.000891
0.00149 (a)
Fluoride
0.665
1.14
0.684
0.684 (e)
0.183
0.183 (e)
2.11 (f)
2.11
2.11 (f)
2.11
Iron
0.115
0.115 (e)
2.73
4.31
0.128
0.128 (e)
1.46 (f)
1.46
0.361 (a)
Lead (g)
0.00569 (b)
0.00569 (b)
0.00569
0.00569 (e)
0.00569 (b)
0.00569 (b)
(d)
(d)
0.00
0.00
Magnesium
2.58 (b)
2.58 (b)
2.58
2.58 (e)
(d)
(d)
(d)
(d)
(d)
(d)
Manganese
0.346
0.346 (e)
0.442 (a)
0.0574
0.0574 (e)
(d)
(d)
0.368
0.368 (e)
Nickel (g)
0.00 (b)
0.00 (b)
0.00
0.00 (e)
(d)
(d)
(d)
(d)
(d)
(d)
9-14

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-4. Long-Term Average Concentrations from DMR Data by Source Water Treatment Type and Residuals Treatment
(mg/L)
Pollutant
Precipitative Softening
Coagulation and
Filtration
Filtration only
Membrane Desalination
Ion Exchange and
Adsorption
Solids
Separation
No Solids
Separation
Solids
Separation
No Solids
Separation
Solids
Separation
No Solids
Separation
Solids
Separation
No Solids
Separation
Solids
Separation
No Solids
Separation
Phosphorus,
Total
0.423 (b)
0.423 (b)
0.423
0.423 (e)
0.423 (b)
0.423 (b)
0.0678 (f)
0.0678
0.0965 (a)
Zinc (g)
0.316 (b)
0.316 (b)
0.316
0.316 (e)
(d)
(d)
(d)
(d)
0.00473
0.00473 (e)
Source: U.S. EPA, 2007
a – EPA calculated average concentration using all plants within the source water treatment type group regardless of residuals treatment in place. The average concentration for
WTPs without solid/water separation was less than the average concentration for WTPs with solid/water separation.
b – Transferred pollutant concentration from coagulation and filtration because no other data were available.
c – DMR data available for ion exchange/membrane desalination plants only. Note that one plant with data also listed coagulation and filtration; however the chlorides load is
expected to be due to ion exchange.
d – No DMR data were available for this pollutant and model plant. EPA did not estimate loadings for this pollutant and model plant.
e – DMR data available only for WTPs that perform solid/water separation. EPA used the treated concentration to estimate untreated pollutant loadings.
f – DMR data available only for WTPs that do not perform solid/water separation. EPA used the untreated concentration to estimate treated pollutant loadings.
g – Percent of non-detect samples in DMR databases exceeds 10 percent. Nickel was not detected above the detection limit for any sample; therefore EPA set the LTA equal to
zero (0).
9-15

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-5. Long-Term Average Concentrations for Pollutants Resulting from Disinfection
with Chlorine
Pollutant
Dechlorination
Performed
No Dechlorination
Performed
Source for
Concentration
Total trihalomethanes
0 mg/L (a)
0.00223 mg/L
DMR Data
Chloroform (CHCl
2
)
0 mg/L (a)
0.050 mg/L
DMR Data
Bromodichloromethane (CHCl
2
Br,
Dichlorobromomethane)
0 mg/L (a)
0.010 mg/L
DMR Data
Bromoform (tribromomethane)
0 mg/L (a)
0.050 mg/L
Transfer from
Chloroform
Dibromochloromethane (CDBM;
Chlorodibromomethane)
0 mg/L (a)
0.002 mg/L
DMR Data
Haloacetic acids (5HAA’s)
0 mg/L (a)
0.060 mg/L
MCL
Total residual chlorine (b)
0.144 mg/L
0.192 mg/L
DMR Data
a – No DMR data were available. EPA assumed that, in WTPs that perform dechlorination, the effluent
concentrations of these parameters are not present (i.e., are zero).
b – Percent of non-detect samples in DMR databases exceeds 10 percent.
9.3.3 DMR Data Limitations
The DMR data received as part of the industry questionnaire includes limitations
which affected the calculation of pollutant loadings estimates. In these cases, EPA used its best
engineering judgment to calculate loadings. The primary data limitation is that there was no
standard list of pollutants monitored by all WTPs. The DMR data submitted by each WTP
includes data for only those pollutants listed in the plant’s NPDES permit. For example, a state
may have a core set of pollutants that WTPs need to monitor; however, additional pollutant
monitoring might be more random and dependent on the watershed characteristics of the source
water. Furthermore, it is not known why pollutants are monitored at specific facilities, that is,
whether the monitoring is due to suspected problems so that these facilities are more likely to be
representative of high loading plants than not. On the other hand, it may be the case that facilities
with lower loading levels were more likely to report their DMR data with the 2007 industry
questionnaire In the absence of additional information, it’s not possible to describe the potential
magnitude and direction of bias, if any. Where appropriate, EPA transferred pollutant
concentrations from another model plant group.
EPA also found that some model plants had more data than others. For example,
EPA had DMR data for 24 precipitative softening plants and for 76 coagulation/filtration plants.
9-16

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Whereas, EPA had a limited number of WTPs with DMR data for the three other source water
treatment types: filtration only (5 WTPs), membrane desalination (2 WTPs), and ion
exchange/adsorption (4 WTPs). For the filtration only WTPs, 4 WTPs represented discharges
following solid/water separation and only one WTP represented untreated discharges. For the ion
exchange/adsorption, 3 WTPs represented discharges following solid/water separation and only
one WTP represented untreated discharges. Neither membrane desalination plants treated the
discharge using solid/water separation prior to discharge. In some cases, only a single WTP had
DMR data for a pollutant in a certain model plant group. EPA used these data to estimate the
pollutant concentration, however, EPA does not have the data available to determine whether the
concentration reported is representative of a majority of discharges for the model plant group.
Membrane desalination plants typically do not treat the concentrate prior to
discharge (Malmrose, et al., 2004). Therefore, EPA used the same pollutant concentration to
represent WTPs treating residuals via solid/water separation as those WTPs not treating residuals
prior to discharge. EPA's survey database included only two membrane desalination plants that
performed solid/water separation. Both of these WTPs are zero discharge plants (U.S. EPA,
2009a).
Where appropriate, EPA transferred concentrations or modified the calculation of
average pollutant concentrations to use for the pollutant loading estimates. The memorandum
entitled, Pollutant Loadings Estimates for Drinking Water Treatment Plants (ERG, 2009),
provides details.
9.4 MODEL PLANT FLOW RATE ESTIMATION
As noted above, EPA determined that the effluent flow rate would vary based on
three of the four model plant characteristics: 1) source water treatment; 2) population served size;
and 3) discharge type. EPA did not distinguish flow rates by residuals treatment type; EPA
assumed that the flow rate would not be significantly altered by solid/water separation or
dechlorination. Solid/water separation results in removal of certain parameters from the
wastewater (e.g., TSS, metals); however EPA does not believe this will result in a significant
difference in the wastewater volume discharged. The dechlorination process is the addition of
9-17

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
sulfur chemicals (e.g., sodium metabisulfite) to react with chlorine in the wastewater and remove
free chlorine and total combined chlorine residual. The addition of the sulfur chemicals is not
expected to significantly impact the wastewater volume discharged.
EPA estimated the model plant flow rates using reported flow data from DMRs
submitted with the 2007 industry questionnaire and the responses to the survey (U.S. EPA, 2007;
U.S. EPA, 2009a). As such, the loadings estimate will be reflective of actual flow rather than
design flow. An additional data limitation for this analysis is that the flow rates from 2006 (either
in response to the 2007 industry questionnaire or included with the 2006 DMR data) might not
be typical.
9.4.1 Review of DMR and Survey Data
EPA categorized each of the 108 WTPs that submitted survey responses and
DMR data into a model plant type to calculate flow rate. EPA used the DMR data to estimate
direct discharge flow rates and survey responses to estimate indirect discharge flow rates and
population served. EPA then used the DMR and survey data to estimate model plant effluent
flow rates. For direct discharging WTPs, EPA assumed continuous discharge (i.e., discharge
occurs 365 days per year).
For indirect dischargers, EPA reviewed responses to the questionnaire to
determine whether the discharge was continuous, batch, or an emergency discharge. For
continuous indirect discharges, EPA assumed the discharge occurred 365 days per year. For
batch discharges, EPA multiplied the volume discharged by the number of batches per year and
then normalized the flow rate to 365 days per year. For example, if a WTP discharged 1,000,000
gallons (1 MG) for 20 days of the year, then the flow rate normalized for the year was calculated
as follows:
(1 MG × 20 DPY)/365 = 0.055 MGD (Eq. 9-4)
If the number of batch discharges was greater than 365 days per year, EPA assumed the
discharge occurred 365 days per year, for the purpose of estimating the average daily discharge
9-18

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
rate. For example, if a WTP reported 1,100 batch discharges annually and each batch was 1 MG,
EPA calculated the discharge as follows:
(1 MG × 1,100 Batches)/365 = 3.0 MGD (Eq. 9-5)
For indirect dischargers reporting both continuous and batch flow rates, EPA summed the two
quantities to estimate total daily flow rate from the WTP. EPA excluded emergency discharge
volumes unless no other discharges (i.e., continuous or batch) were reported.
9.4.2 Model Plant Effluent Flow Rate Results
For each model plant group, EPA calculated an average effluent flow rate using
the data from individual WTPs. Table 9-6 presents the model plant flow rates (range of
individual WTP flow rates and average) estimated for each source water treatment type,
population served, and discharge type.
Table 9-6. Model Plant Effluent Flow Rates
Treatment Plant
Type
Population
Served
Direct
Discharge
Effluent Flow
Rate Range
(MGD)
Direct
Discharge
Average
Effluent
(MGD)
Indirect
Discharge
Effluent Flow
Rate Range
(MGD) (a)
Indirect
Discharge
Average Effluent
(MGD) (a)
Precipitative
Softening
10,001 to 50,000
0.04175 to
0.432
0.235
0.00021 to 0.469
0.144
50,001 to 100,000
0.062 to 0.512
0.312
0.00082 to 0.830
0.297
100,001 to
500,000
0.067 to 20.1
3.79
0.00091 to 1.057
0.339
>500,000
3.56 to 7.79
5.68
(b)
0.339
Coagulation &
Filtration
10,001 to 50,000
0.0114 to 0.903
0.209
4.5E-7 to 0.61
0.089
50,001 to 100,000
0.003 to 1.26
0.376
4.5E-6 to 1.1
0.168
100,001 to
500,000
0.0046 to 3.5
1.22
0.000146 to 3.13
0.276
>500,000
0.502 to 7.04
3.47
0.0142 to 0.985
0.291
Filtration Only
10,001 to 50,000
0.0656 to 0.337
0.179
8.6E-7 to 0.18
0.040
50,001 to 100,000
(b)
0.179
0.00063 to
0.341(d)
0.171(d)
100,001 to
500,000
0.734 to 1.36
1.05
0.00063 to
0.341(d)
0.171(d)
>500,000
(b)
1.05
(b)
0.171
9-19

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-6. Model Plant Effluent Flow Rates
Treatment Plant
Type
Population
Served
Direct
Discharge
Effluent Flow
Rate Range
(MGD)
Direct
Discharge
Average
Effluent
(MGD)
Indirect
Discharge
Effluent Flow
Rate Range
(MGD) (a)
Indirect
Discharge
Average Effluent
(MGD) (a)
Membrane
Desalination
10,001 to 50,000
(c)
0.627
1.5E-5 to 0.00274
0.002
50,001 to 100,000
(c)
1.15
(c)
0.26
100,001 to
500,000
(b)
1.15
0.122 to 0.997
0.560
>500,000
(f)
Not applicable
(f)
Not applicable
Ion Exchange &
Adsorption
10,001 to 50,000
0.0576 to 0.185
0.120
2.8E-6 to 0.7(e)
0.110(e)
50,001 to 100,000
(c)
1.15
2.8E-6 to 0.7(e)
0.110(e)
100,001 to
500,000
(f)
Not applicable
(b)
0.110
>500,000
(f)
Not applicable
(f)
Not applicable
Sources: U.S. EPA, 2007; U.S. EPA, 2009a
MGD – Million Gallons per Day
a – EPA calculated annual normalized flow rates using indirect discharge data from the survey response database for
the 2006 industry questionnaire (U.S. EPA, 2008a). EPA multiplied the gallons per day by the number of days per
year reported in the survey; and then divided by 365 days per year.
b – No flow rate data were available for this population category. For pollutant loadings analysis, EPA transferred
the average flow rate from the next smallest population group with the same treatment plant type.
c – Not applicable: only one WTP falls into this characteristic group.
d – For the indirect discharge effluent flow rate, EPA combined the flow rate averages for plants serving 50,001 to
100,000 people with the flow rate averages for plants serving between 100,001 and 500,000. The average flow rate
for the larger population group is smaller than the average flow rate for the smaller population group suggesting that
this size distinction is not adequately represented or less meaningful.
e – For the indirect discharge effluent flow rate, EPA combined the flow rate averages for plants serving 10,001 to
50,000 people with the flow rate averages for plants serving between 50,001 and 100,000. The average flow rate for
the larger population group is smaller than the average flow rate for the smaller population group suggesting that
this size distinction is not adequately represented or less meaningful.
f – The national estimates do not include any WTPs in this population category.
9.5 RESULTS OF THE POLLUTANT LOADINGS ESTIMATE FOR MODEL
PLANTS
EPA calculated the pollutant loadings and TWPE for each model plant as
described above. EPA did not have data to quantify the pollutant discharges attributed to source
water contaminants and those attributed to treatment chemical addition. The portion from source
water contaminants would be site-specific; WTPs did not submit source water quality data to pair
with the effluent discharge data. However, WTPs might collect source water quality data to help
optimize addition of treatment chemicals. These data can be used by permit writers when
developing best professional judgment (BPJ) permit limitations. From literature data, membrane
9-20

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
concentrate has very few process-added chemicals and the pollutants are primarily from the
source water (U.S. EPA, ASCE, AWWA, 1996).
Tables 9-7 through 9-10 each show the pollutant loading estimate for model
plants by the five source water treatment types and by residuals treatment type (with or without
solid/water separation) for direct and indirect dischargers. Each table is for a different population
served size category.
Table 9-7. Model Plant Pollutant Loadings by Source Water Treatment Type and
Residuals Treatment Type (With and Without Solid/Water Separation) for Direct and
Indirect (Pass Through) Discharges: Population Served of 10,001 to 50,000 People
Source Water Treatment
Type and Pollutant
Pollutant Loadings for Model Plants Serving 10,001 to 50,000 People
With Solid/Water Separation
Without Solid/Water Separation
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Precipitative Softening
Bulk Parameters
BOD
1030
68.8
1030
68.8
Nitrogen, Total
2610
679
2610
679
TSS
4220
270
1030000
65600
Total Bulk Parameters
(Precipitative Softening)
7860
1017.8
1033640
66347.8
Specific Parameters
Aluminum
127
8.2
6.98
0.452
127
8.2
6.98
0.452
Ammonia
345
0.383
129
0.143
345
0.383
129
0.143
Barium
7.16
0.0143
1.96
0.00391
7.16
0.0143
1.96
0.00391
Calcium
6250
0.175
3500
0.0979
6250
0.175
3500
0.0979
Copper
49.6
31.5
4.8
3.04
49.6
31.5
4.8
3.04
Fluoride
476
16.7
113
3.94
814
28.5
192
6.74
Iron
82.3
0.461
9.07
0.0508
82.3
0.461
9.07
0.0508
Lead
4.07
9.12
0.562
1.26
4.07
9.12
0.562
1.26
Magnesium
1840
1.6
968
0.838
1840
1.6
968
0.838
Manganese
248
17.5
90.1
6.34
248
17.5
90.1
6.34
Nickel
0
0
0
0
0
0
0
0
Phosphorus, Total
303
57.4
303
57.4
Zinc
226
10.6
28.9
1.35
226
10.6
28.9
1.35
Total Specific Parameters
(Precipitative Softening)
9,958
96
4,910
18
10,296
108
4,989
20
9-21

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-7. Model Plant Pollutant Loadings by Source Water Treatment Type and
Residuals Treatment Type (With and Without Solid/Water Separation) for Direct and
Indirect (Pass Through) Discharges: Population Served of 10,001 to 50,000 People
Source Water Treatment
Type and Pollutant
Pollutant Loadings for Model Plants Serving 10,001 to 50,000 People
With Solid/Water Separation
Without Solid/Water Separation
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Coagulation & Filtration
Bulk Parameters
BOD
921
42.8
921
42.8
Nitrogen, Total
2320
422
2320
422
TSS
34700
1550
85800
3830
Total Bulk Parameters
(Coagulation & Filtration)
37941
2014.8
89041
4294.8
Specific Parameters
Aluminum
1380
89
52.9
3.42
1380
89
52.9
3.42
Ammonia
308
0.341
80.2
0.089
308
0.341
80.2
0.089
Barium
6.38
0.0127
1.22
0.00243
6.38
0.0127
1.22
0.00243
Calcium
5570
0.156
2170
0.0609
5570
0.156
2170
0.0609
Copper
44.2
28.1
2.98
1.89
44.2
28.1
2.98
1.89
Fluoride
436
15.3
72.1
2.52
436
15.3
72.1
2.52
Iron
1740
9.76
134
0.751
2750
15.4
211
1.18
Lead
3.63
8.13
0.349
0.783
3.63
8.13
0.349
0.783
Magnesium
1640
1.42
602
0.521
1640
1.42
602
0.521
Manganese
282
19.8
71.5
5.03
282
19.8
71.5
5.03
Nickel
0
0
0
0
0
0
0
0
Phosphorus, Total
270
35.7
270
35.7
Zinc
201
9.45
18
0.842
201
9.45
18
0.842
Total Specific Parameters
(Coagulation & Filtration)
11,881
181
3,241
16
12,891
187
3,318
16
Filtration Only
Bulk Parameters
BOD
788
19.1
788
19.1
Nitrogen, Total
1990
188
1990
188
TSS
1430
33.2
12200
285
Total Bulk Parameters
(Filtration Only)
4208
240.3
14978
492.1
9-22

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-7. Model Plant Pollutant Loadings by Source Water Treatment Type and
Residuals Treatment Type (With and Without Solid/Water Separation) for Direct and
Indirect (Pass Through) Discharges: Population Served of 10,001 to 50,000 People
Source Water Treatment
Type and Pollutant
Pollutant Loadings for Model Plants Serving 10,001 to 50,000 People
With Solid/Water Separation
Without Solid/Water Separation
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Specific Parameters
Aluminum
501
32.4
10
0.649
501
32.4
10
0.649
Ammonia
263
0.292
35.7
0.0396
263
0.292
35.7
0.0396
Fluoride
100
3.5
8.59
0.301
100
3.5
8.59
0.301
Iron
69.9
0.392
2.8
0.0157
69.9
0.392
2.8
0.0157
Lead
3.1
6.95
0.156
0.348
3.1
6.95
0.156
0.348
Manganese
31.3
2.2
4.13
0.291
31.3
2.2
4.13
0.291
Phosphorus, Total
231
15.9
231
15.9
Total Specific Parameters
(Filtration Only)
1,199
46
77
2
1,199
46
77
2
Membrane Desalination
Bulk Parameters
CBOD5
1910
0.52
1910
0.52
Nitrogen, Total
5640
6.01
5640
6.01
TSS
5460
1.43
5460
1.43
Total Bulk Parameters
(Membrane Desalination)
13010
7.96
13010
7.96
Specific Parameters
Ammonia
2960
3.29
4.52
0.00501
2960
3.29
4.52
0.00501
Cadmium
1.98
45.7
0.000492
0.0114
1.98
45.7
0.000492
0.0114
Chlorides
13600000
331
14500
0.353
13600000
331
14500
0.353
Copper
1.7
1.08
0.000673
0.000427
1.7
1.08
0.000673
0.000427
Fluoride
4030
141
3.89
0.136
4030
141
3.89
0.136
Iron
2800
15.7
1.26
0.00705
2800
15.7
1.26
0.00705
Phosphorus, Total
129
0.1
129
0.1
Total Specific Parameters
(Membrane Desalination)
13,609,923
538
14,510
1
13,609,923
538
14,510
1
Ion Exchange and Adsorption
Bulk Parameters
CBOD5
366
36.4
366
36.4
Nitrogen, Total
172
67.2
332
129
TDS
3130000
2640000
3130000
2640000
TSS
2330
223
2330
223
Total Bulk Parameters
(Ion Exchange &
Adsorption)
3132868
2640326.6
3133028
2640388.4
9-23

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-7. Model Plant Pollutant Loadings by Source Water Treatment Type and
Residuals Treatment Type (With and Without Solid/Water Separation) for Direct and
Indirect (Pass Through) Discharges: Population Served of 10,001 to 50,000 People
Source Water Treatment
Type and Pollutant
Pollutant Loadings for Model Plants Serving 10,001 to 50,000 People
With Solid/Water Separation
Without Solid/Water Separation
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Specific Parameters
Ammonia
32.7
0.0363
18.2
0.0203
32.7
0.0363
18.2
0.0203
Cadmium
0.378
8.75
0.0345
0.797
0.378
8.75
0.0345
0.797
Chlorides
1070000
26
417000
10.2
2600000
63.3
1010000
24.7
Copper
0.544
0.345
0.0787
0.0499
0.544
0.345
0.0787
0.0499
Fluoride
771
27
273
9.55
771
27
273
9.55
Iron
132
0.74
21.8
0.122
132
0.74
21.8
0.122
Lead
0
0
0
0
0
0
0
0
Manganese
134
9.47
73.1
5.15
134
9.47
73.1
5.15
Phosphorus, Total
35.3
10
35.3
10
Zinc
1.73
0.081
0.33
0.0155
1.73
0.081
0.33
0.0155
Total Specific Parameters
(Ion Exchange &
Adsorption)
1,071,108
72
417,397
26
2,601,108
110
1,010,397
40
Source: U.S. EPA, 2008a; U.S. EPA, 2009b
Blanks indicate that for this pollutant, no TWF is available and therefore, no TWPE were calculated. EPA does not derive TWFs
for conventional pollutants.
Zero indicates that EPA estimates the load for this pollutant at zero lbs/yr.
Table 9-8. Model Plant Pollutant Loadings by Source Water Treatment Type and
Residuals Treatment Type (With and Without Solid/Water Separation) for Direct and
Indirect (Pass Through) Discharges: Population Served of 50,001 to 100,000 People
Source Water Treatment
Type and Pollutant
Pollutant Loadings for Model Plants Serving 50,001 to 100,000 People
With Solid/Water Separation
Without Solid/Water Separation
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-
eq/yr)
Precipitative Softening
Bulk Parameters
BOD
1370
142
1370
142
Nitrogen, Total
3460
1400
3460
1400
TSS
5610
556
1360000
135000
Total Bulk Parameters
(Precipitative Softening)
10440
2098
1364830
136542
9-24

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-8. Model Plant Pollutant Loadings by Source Water Treatment Type and
Residuals Treatment Type (With and Without Solid/Water Separation) for Direct and
Indirect (Pass Through) Discharges: Population Served of 50,001 to 100,000 People
Source Water Treatment
Type and Pollutant
Pollutant Loadings for Model Plants Serving 50,001 to 100,000 People
With Solid/Water Separation
Without Solid/Water Separation
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-
eq/yr)
Specific Parameters
Aluminum
168
10.9
14.4
0.932
168
10.9
14.4
0.932
Ammonia
459
0.509
266
0.295
459
0.509
266
0.295
Barium
9.52
0.0189
4.05
0.00807
9.52
0.0189
4.05
0.00807
Calcium
8300
0.232
7210
0.202
8300
0.232
7210
0.202
Copper
65.9
41.8
9.89
6.28
65.9
41.8
9.89
6.28
Fluoride
633
22.1
232
8.13
1080
37.9
397
13.9
Iron
109
0.613
18.7
0.105
109
0.613
18.7
0.105
Lead
5.41
12.1
1.16
2.6
5.41
12.1
1.16
2.6
Magnesium
2450
2.12
2000
1.73
2450
2.12
2000
1.73
Manganese
329
23.2
186
13.1
329
23.2
186
13.1
Nickel
0
0
0
0
0
0
0
0
Phosphorus, Total
402
119
402
119
Zinc
301
14.1
59.5
2.79
301
14.1
59.5
2.79
Total Specific Parameters
(Precipitative Softening)
13,232
128
10,121
36
13,679
143
10,286
42
Coagulation and Filtration
Bulk Parameters
BOD
1650
80.3
1650
80.3
Nitrogen, Total
4170
792
4170
792
TSS
62400
2910
154000
7180
Total Bulk Parameters
(Coagulation and Filtration)
68220
3782.3
159820
8052.3
Specific Parameters
Aluminum
2470
160
99.2
6.41
2470
160
99.2
6.41
Ammonia
553
0.613
150
0.167
553
0.613
150
0.167
Barium
11.5
0.0228
2.29
0.00456
11.5
0.0228
2.29
0.00456
Calcium
10000
0.28
4080
0.114
10000
0.28
4080
0.114
Copper
79.4
50.4
5.59
3.55
79.4
50.4
5.59
3.55
Fluoride
784
27.4
135
4.73
784
27.4
135
4.73
Iron
3130
17.5
251
1.41
4940
27.6
396
2.22
Lead
6.52
14.6
0.655
1.47
6.52
14.6
0.655
1.47
Magnesium
2950
2.55
1130
0.978
2950
2.55
1130
0.978
9-25

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-8. Model Plant Pollutant Loadings by Source Water Treatment Type and
Residuals Treatment Type (With and Without Solid/Water Separation) for Direct and
Indirect (Pass Through) Discharges: Population Served of 50,001 to 100,000 People
Source Water Treatment
Type and Pollutant
Pollutant Loadings for Model Plants Serving 50,001 to 100,000 People
With Solid/Water Separation
Without Solid/Water Separation
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-
eq/yr)
Manganese
506
35.6
134
9.44
506
35.6
134
9.44
Nickel
0
0
0
0
0
0
0
0
Phosphorus, Total
485
67
485
67
Zinc
362
17
33.7
1.58
362
17
33.7
1.58
Total Specific Parameters
(Coagulation & Filtration)
21,337
326
6,088
30
23,147
336
6,233
31
Filtration Only
Bulk Parameters
BOD
788
81.8
788
81.8
Nitrogen, Total
1990
807
1990
807
TSS
1430
142
12200
1220
Total Bulk Parameters
(Filtration Only)
4208
1030.8
14978
2108.8
Specific Parameters
Aluminum
501
32.4
43
2.78
501
32.4
43
2.78
Ammonia
263
0.292
153
0.17
263
0.292
153
0.17
Fluoride
100
3.5
36.9
1.29
100
3.5
36.9
1.29
Iron
69.9
0.392
12
0.0673
69.9
0.392
12
0.0673
Lead
3.1
6.95
0.667
1.49
3.1
6.95
0.667
1.49
Manganese
31.3
2.2
17.7
1.25
31.3
2.2
17.7
1.25
Phosphorus, Total
231
68.2
231
68.2
Total Specific Parameters
(Filtration Only)
1,199
46
331
7
1,199
46
331
7
Membrane Desalination
Bulk Parameters
CBOD5
3500
86.2
3500
86.2
Nitrogen, Total
10300
996
10300
996
TSS
10000
237
10000
237
Total Bulk Parameters
(Membrane Desalination)
23800
1319.2
23800
1319.2
9-26

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-8. Model Plant Pollutant Loadings by Source Water Treatment Type and
Residuals Treatment Type (With and Without Solid/Water Separation) for Direct and
Indirect (Pass Through) Discharges: Population Served of 50,001 to 100,000 People
Source Water Treatment
Type and Pollutant
Pollutant Loadings for Model Plants Serving 50,001 to 100,000 People
With Solid/Water Separation
Without Solid/Water Separation
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-
eq/yr)
Specific Parameters
Ammonia
5430
6.03
749
0.831
5430
6.03
749
0.831
Cadmium
3.63
83.8
0.0816
1.89
3.63
83.8
0.0816
1.89
Chlorides
24900000
607
2400000
58.4
24900000
607
2400000
58.4
Copper
3.12
1.98
0.111
0.0708
3.12
1.98
0.111
0.0708
Fluoride
7390
259
646
22.6
7390
259
646
22.6
Iron
5130
28.7
209
1.17
5130
28.7
209
1.17
Phosphorus, Total
237
16.6
237
16.6
Total Specific Parameters
(Membrane Desalination)
24,918,194
987
2,401,621
85
24,918,194
987
2,401,621
85
Ion Exchange & Adsorption
Bulk Parameters
CBOD5
3500
36.4
3500
36.4
Nitrogen, Total
1650
67.2
3180
129
TDS
30000000
2640000
30000000
2640000
TSS
22300
223
22300
223
Total Bulk Parameters (Ion
Exchange & Adsorption)
30027450
2640326.6
30028980
2640388.4
Specific Parameters
Ammonia
313
0.348
18.2
0.0203
313
0.348
18.2
0.0203
Cadmium
3.63
83.8
0.0345
0.797
3.63
83.8
0.0345
0.797
Chlorides
10200000
250
417000
10.2
24900000
607
1010000
24.7
Copper
5.21
3.31
0.0787
0.0499
5.21
3.31
0.0787
0.0499
Fluoride
7390
259
273
9.55
7390
259
273
9.55
Iron
1270
7.09
21.8
0.122
1270
7.09
21.8
0.122
Lead
0
0
0
0
0
0
0
0
Manganese
1290
90.7
73.1
5.15
1290
90.7
73.1
5.15
Phosphorus, Total
338
10
338
10
Zinc
16.6
0.777
0.33
0.0155
16.6
0.777
0.33
0.0155
Total Specific Parameters
(Ion Exchange &
Adsorption)
10,210,626
695
417,397
26
24,910,626
1,052
1,010,397
40
Source: U.S. EPA, 2008a; U.S. EPA, 2009b
Blanks indicate that for this pollutant, no TWF is available and therefore, no TWPE were calculated. EPA does not derive TWFs
for conventional pollutants.
Zero indicates that EPA estimates the load for this pollutant at zero lbs/yr.
a – Excluded from total: chlorides is a constituent of TDS.
9-27

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-9. Model Plant Pollutant Loadings by Source Water Treatment Type and
Residuals Treatment Type (With and Without Solid/Water Separation) for Direct and
Indirect (Pass Through) Discharges: Population Served of 100,001 to 500,000 People
Source Water Treatment
Type and Pollutant
Pollutant Loadings for Model Plants Serving 100,001 to 500,000 People
With Solid/Water Separation
Without Solid/Water Separation
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Precipitative Softening
Bulk Parameters
BOD
16,700
162
16,700
162
Nitrogen, Total
42,100
1,600
42,100
1,600
TSS
68,100
636
16,600,000
155,000
Total Bulk Parameters
(Precipitative Softening)
126,900
2,398
16,658,800
156,762
Specific Parameters
Aluminum
2,050
132
16.4
1.06
2,050
132
16.4
1.06
Ammonia
5,570
6.19
304
0.337
5,570
6.19
304
0.337
Barium
116
0.23
4.63
0.00922
116
0.23
4.63
0.00922
Calcium
101,000
2.82
8240
0.231
101,000
2.82
8,240
0.231
Copper
801
508
11.3
7.17
801
508
11.3
7.17
Fluoride
7,690
269
265
9.29
13,100
460
454
15.9
Iron
1,330
7.44
21.4
0.12
1,330
7.44
21.4
0.12
Lead
65.7
147
1.32
2.97
65.7
147
1.32
2.97
Magnesium
29,800
25.8
2280
1.98
29,800
25.8
2,280
1.98
Manganese
4,000
282
212
15
4,000
282
212
15
Nickel
0
0
0
0
0
0
0
0
Phosphorus, Total
4,890
135
4,890
135
Zinc
3,650
171
68
3.19
3,650
171
68
3.19
Total Specific Parameters
(Precipitative Softening)
160,963
1,551
11,559
41
166,373
1,742
11,748
48
Coagulation & Filtration
Bulk Parameters
BOD
5,350
132
5,350
132
Nitrogen, Total
13,500
1,300
13,500
1,300
TSS
202,000
4,790
498,000
11,800
Total Bulk Parameters
(Coagulation & Filtration)
220,850
6,222
516,850
13,232
9-28

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-9. Model Plant Pollutant Loadings by Source Water Treatment Type and
Residuals Treatment Type (With and Without Solid/Water Separation) for Direct and
Indirect (Pass Through) Discharges: Population Served of 100,001 to 500,000 People
Source Water Treatment
Type and Pollutant
Pollutant Loadings for Model Plants Serving 100,001 to 500,000 People
With Solid/Water Separation
Without Solid/Water Separation
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Specific Parameters
Aluminum
7,980
516
163
10.6
7,980
516
163
10.6
Ammonia
1,780
1.98
248
0.275
1,780
1.98
248
0.275
Barium
37
0.0737
3.77
0.00751
37
0.0737
3.77
0.00751
Calcium
32,300
0.904
6,720
0.188
32,300
0.904
6,720
0.188
Copper
256
163
9.21
5.85
256
163
9.21
5.85
Fluoride
2,530
88.6
223
7.79
2,530
88.6
223
7.79
Iron
10,100
56.6
414
2.32
15,900
89.3
653
3.66
Lead
21.1
47.2
1.08
2.42
21.1
47.2
1.08
2.42
Magnesium
9,530
8.25
1,860
1.61
9,530
8.25
1,860
1.61
Manganese
1,630
115
221
15.5
1,630
115
221
15.5
Nickel
0
0
0
0
0
0
0
0
Phosphorus, Total
1,570
110
1,570
110
Zinc
1,170
54.8
55.5
2.6
1,170
54.8
55.5
2.6
Total Specific Parameters
(Coagulation & Filtration)
68,904
1,052
10,029
49
74,704
1,085
10,268
51
Filtration Only
Bulk Parameters
BOD
4,610
81.8
4,610
81.8
Nitrogen, Total
11,600
807
11,600
807
TSS
8,360
142
71,700
1,220
Total Bulk Parameters
(Filtration Only)
24,570
1,031
87,910
2,109
Specific Parameters
Aluminum
2,930
190
43
2.78
2,930
190
43
2.78
Ammonia
1,540
1.71
153
0.17
1,540
1.71
153
0.17
Fluoride
585
20.5
36.9
1.29
585
20.5
36.9
1.29
Iron
409
2.29
12
0.0673
409
2.29
12
0.0673
Lead
18.2
40.7
0.667
1.49
18.2
40.7
0.667
1.49
Manganese
183
12.9
17.7
1.25
183
12.9
17.7
1.25
Phosphorus, Total
1,350
68.2
1,350
68.2
Total Specific Parameters
(Filtration Only)
7,015
268
331
7
7,015
268
331
7
9-29

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-9. Model Plant Pollutant Loadings by Source Water Treatment Type and
Residuals Treatment Type (With and Without Solid/Water Separation) for Direct and
Indirect (Pass Through) Discharges: Population Served of 100,001 to 500,000 People
Source Water Treatment
Type and Pollutant
Pollutant Loadings for Model Plants Serving 100,001 to 500,000 People
With Solid/Water Separation
Without Solid/Water Separation
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Membrane Desalination
Bulk Parameters
CBOD5
3,500
185
3,500
185
Nitrogen, Total
10,300
2,140
10,300
2,140
TSS
10,000
509
10,000
509
Total Bulk Parameters
(Membrane Desalination)
23,800
2,834
23,800
2,834
Specific Parameters
Ammonia
5,430
6.03
1,610
1.79
5,430
6.03
1,610
1.79
Cadmium
3.63
83.8
0.176
4.06
3.63
83.8
0.176
4.06
Chlorides
24,900,000
607
5,170,000
126
24,900,000
607
5,170,000
126
Copper
3.12
1.98
0.24
0.152
3.12
1.98
0.24
0.152
Fluoride
7,390
259
1,390
48.6
7,390
259
1,390
48.6
Iron
5,130
28.7
449
2.52
5,130
28.7
449
2.52
Phosphorus, Total
237
35.8
237
35.8
Total Specific Parameters
(Membrane Desalination)
24,918,194
987
5,173,485
183
24,918,194
987
5,173,485
183
Ion Exchange & Adsorption
Bulk Parameters
CBOD5
Not applicable. No
plants in this model
plant group.
36.4
Not applicable. No plants in this model plant
group.
Nitrogen, Total
67.2
TDS
2,640,000
TSS
223
Total Bulk Parameters (Ion
Exchange & Adsorption)
2,640,327
Specific Parameters
Ammonia
Not applicable. No
plants in this model
plant group.
18.2
0.0203
Not applicable. No plants in this model plant
group.
Cadmium
0.0345
0.797
Chlorides
417,000
10.2
Copper
0.0787
0.0499
Fluoride
273
9.55
Iron
21.8
0.122
Lead
0
0
Manganese
73.1
5.15
Phosphorus, Total
10
Zinc
0.33
0.0155
Total Specific Parameters
(Ion Exchange &
Adsorption)
417,397
26
9-30

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Source: U.S. EPA, 2008a; U.S. EPA, 2009b
Blanks indicate that for this pollutant, no TWF is available and therefore, no TWPE were calculated. EPA does not derive TWFs
for conventional pollutants.
Zero indicates that EPA estimates the load for this pollutant at zero lbs/yr.
Table 9-10. Model Plant Pollutant Loadings by Source Water Treatment Type and
Residuals Treatment Type (With and Without Solid/Water Separation) for Direct and
Indirect (Pass Through) Discharges: Population Served of More than 500,000 People
Source Water Treatment
Type and Pollutant
Pollutant Loadings for Model Plants Serving >500,000 People
With Solid/Water Separation
Without Solid/Water Separation
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Precipitative Softening
Bulk Parameters
BOD
25,000
162
25,000
Not applicable. No
plants in this model
plant group.
Nitrogen, Total
62,900
1,600
62,900
TSS
102,000
636
24,800,00
0
Total Bulk Parameters
(Precipitative Softening)
189,900
2,398
24,887,90
0
Specific Parameters
Aluminum
3,060
198
16.4
1.06
3,060
198
Not applicable. No
plants in this model
plant group.
Ammonia
8,340
9.25
304
0.337
8,340
9.25
Barium
173
0.344
4.63
0.00922
173
0.344
Calcium
151,000
4.22
8240
0.231
151,000
4.22
Copper
1,200
760
11.3
7.17
1,200
760
Fluoride
11,500
402
265
9.29
19,600
688
Iron
1,990
11.1
21.4
0.12
1,990
11.1
Lead
98.3
220
1.32
2.97
98.3
220
Magnesium
44,500
38.5
2280
1.98
44,500
38.5
Manganese
5,980
421
212
15
5,980
421
Nickel
0
0
0
0
0
0
Phosphorus, Total
7,310
135
7,310
Zinc
5,460
256
68
3.19
5,460
256
Total Specific Parameters
(Precipitative Softening)
240,611
2,320
11,559
41
248,711
2,606
Coagulation & Filtration
Bulk Parameters
BOD
15,200
139
15,200
139
Nitrogen, Total
38,400
1,370
38,400
1,370
TSS
575,000
5,040
1,420,000
12,400
Total Bulk Parameters
(Coagulation & Filtration)
628,600
6,549
1,473,600
13,909
9-31

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-10. Model Plant Pollutant Loadings by Source Water Treatment Type and
Residuals Treatment Type (With and Without Solid/Water Separation) for Direct and
Indirect (Pass Through) Discharges: Population Served of More than 500,000 People
Source Water Treatment
Type and Pollutant
Pollutant Loadings for Model Plants Serving >500,000 People
With Solid/Water Separation
Without Solid/Water Separation
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Specific Parameters
Aluminum
22,800
1470
172
11.1
22,800
1,470
172
11.1
Ammonia
5,090
5.65
260
0.289
5,090
5.65
260
0.289
Barium
106
0.21
4
0.0079
106
0.21
3.97
0.0079
Calcium
92,100
2.58
7,070
0.198
92,100
2.58
7,070
0.198
Copper
731
464
9.69
6.15
731
464
9.69
6.15
Fluoride
7,220
253
234
8.19
7,220
253
234
8.19
Iron
28,900
162
436
2.44
45,500
255
687
3.85
Lead
60
135
1.14
2.54
60
135
1.14
2.54
Magnesium
27,200
23.5
1,960
1.69
27,200
23.5
1,960
1.69
Manganese
4,660
328
232
16.4
4,660
328
232
16.4
Nickel
0
0
0
0
0
0
0
0
Phosphorus, Total
4,470
116
4,470
116
Zinc
3,330
156
58.3
2.73
3,330
156
58.3
2.73
Total Specific Parameters
(Coagulation & Filtration)
196,667
3,000
10,553
52
213,267
3,093
10,804
53
Filtration Only
Bulk Parameters
BOD
4,610
81.8
4,610
Not applicable. No
plants in this model
plant group.
Nitrogen, Total
11,600
807
11,600
TSS
8,360
142
71,700
Total Bulk Parameters
(Filtration Only)
24,570
1,031
87,910
Specific Parameters
Aluminum
2,930
190
43
2.78
2,930
190
Not applicable. No
plants in this model
plant group.
Ammonia
1,540
1.71
153
0.17
1,540
1.71
Fluoride
585
20.5
36.9
1.29
585
20.5
Iron
409
2.29
12
0.0673
409
2.29
Lead
18.2
40.7
0.667
1.49
18.2
40.7
Manganese
183
12.9
17.7
1.25
183
12.9
Phosphorus, Total
1,350
68.2
1,350
Total Specific Parameters
(Filtration Only)
7,015
268
331
7
7,015
268
9-32

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-10. Model Plant Pollutant Loadings by Source Water Treatment Type and
Residuals Treatment Type (With and Without Solid/Water Separation) for Direct and
Indirect (Pass Through) Discharges: Population Served of More than 500,000 People
Source Water Treatment
Type and Pollutant
Pollutant Loadings for Model Plants Serving >500,000 People
With Solid/Water Separation
Without Solid/Water Separation
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Membrane Desalination
Not applicable. No plants in this model plant group.
Ion Exchange & Adsorption
Not applicable. No plants in this model plant group.
Source: U.S. EPA, 2008a; U.S. EPA, 2009b
Blanks indicate that for this pollutant, no TWF is available and therefore, no TWPE were calculated. EPA does not derive TWFs
for conventional pollutants.
Zero indicates that EPA estimates the load for this pollutant at zero lbs/yr.
Tables 9-11 and 9-12 each show the pollutant loading estimate for model plants
that disinfect using chlorine by the five source water treatment types and by residuals treatment
type (with or without dechlorination) for direct and indirect dischargers. Table 9-12 shows the
estimate for the population served size categories 10,001 to 50,000 and 50,001 to 100,000. Table
9-13 shows the estimate for the population served size categories 100,001 to 500,000 and greater
than 500,000. EPA did not include any bulk parameters (i.e., parameters that measure more than
one chemical) in the list of pollutants in wastewaters from WTPs that disinfect using chlorine.
9-33

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-11. Model Plant Pollutant Loadings for WTPs Performing Chlorination by Source Water Treatment Type and Residuals
Treatment Type (With and Without Dechlorination) for Direct and Indirect (Pass Through) Discharges: Population Served of 10,001 to
100,000 People
Source Water
Treatment Type and
Pollutant
Pollutant Loadings for Model Plants Serving 10,001 to 50,000 People
Pollutant Loadings for Model Plants Serving 50,001 to 100,000 People
With Dechlorination
Without Dechlorination
With Dechlorination
Without Dechlorination
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE (lb-
eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Precipitative Softening
Total trihalomethanes
0
0
1.6
0.264
0
0
2.12
0.545
Chloroform
0
0
0
0
36.1
0.0751
5.96
0.0124
0
0
0
0
48
0.0997
12.3
0.0256
Bromodichloromethane
0
0
0
0
7.29
0.24
1.59
0.0525
0
0
0
0
9.69
0.319
3.29
0.108
Bromoform
0
0
36.1
5.96
0
0
48
12.3
Dibromochloromethane
0
0
0
0
1.57
0.0699
0.955
0.0425
0
0
0
0
2.09
0.0929
1.97
0.0876
Haloacetic acids
(5HAA’s)
0
0
43
26.3
0
0
57.1
54.2
Total residual chlorine
103
52.6
0
0
137
70
0
0
137
69.9
0
0
183
93
0
0
Total (Precipitative
Softening) (a)
103
52.6
0
0
261.06
70.385
40.765
0.1074
137
69.9
0
0
347.88
93.5116
84.06
0.2212
Coagulation and Filtration
Total trihalomethanes
0
0
1.42
0.164
0
0
2.56
0.308
Chloroform
0
0
0
0
32.2
0.0669
3.71
0.00771
0
0
0
0
57.8
0.12
6.95
0.0145
Bromodichloromethane
0
0
0
0
6.49
0.214
0.992
0.0327
0
0
0
0
11.7
0.384
1.86
0.0612
Bromoform
0
0
32.2
3.71
0
0
57.8
6.95
Dibromochloromethane
0
0
0
0
1.4
0.0623
0.594
0.0264
0
0
0
0
2.52
0.112
1.11
0.0495
Haloacetic acids
(5HAA’s)
0
0
38.3
16.3
0
0
68.7
30.6
Total residual chlorine
92
46.9
0
0
122
62.3
0
0
165
84.2
0
0
220
112
0
0
Total (Coagulation &
Filtration) (a)
92
46.9
0
0
232.59
62.6432
25.306
0.06681
165
84.2
0
0
418.52
112.616
47.47
0.1252
9-34

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-11. Model Plant Pollutant Loadings for WTPs Performing Chlorination by Source Water Treatment Type and Residuals
Treatment Type (With and Without Dechlorination) for Direct and Indirect (Pass Through) Discharges: Population Served of 10,001 to
100,000 People
Source Water
Treatment Type and
Pollutant
Pollutant Loadings for Model Plants Serving 10,001 to 50,000 People
Pollutant Loadings for Model Plants Serving 50,001 to 100,000 People
With Dechlorination
Without Dechlorination
With Dechlorination
Without Dechlorination
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE (lb-
eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Filtration Only
Total trihalomethanes
0
0
1.22
0.0731
0
0
1.22
0.314
Chloroform
0
0
0
0
27.5
0.0572
1.65
0.00343
0
0
0
0
27.5
0.0572
7.08
0.0147
Bromodichloromethane
0
0
0
0
5.55
0.183
0.442
0.0145
0
0
0
0
5.55
0.183
1.89
0.0624
Bromoform
0
0
27.5
1.65
0
0
27.5
7.08
Dibromochloromethane
0
0
0
0
1.2
0.0533
0.264
0.0118
0
0
0
0
1.2
0.0533
1.13
0.0504
Haloacetic acids
(5HAA’s)
0
0
32.7
7.28
0
0
32.7
31.2
Total residual chlorine
78.7
40.1
0
0
105
53.3
0
0
78.7
40.1
0
0
105
53.3
0
0
Total (Filtration
Only) (a)
78.7
40.1
0
0
199.45
53.5935
11.286
0.02973
78.7
40.1
0
0
199.45
53.5935
48.38
0.1275
Membrane Desalination
Total trihalomethanes
0
0
4.26
0.00288
0
0
7.82
0.477
Chloroform
0
0
0
0
96.3
0.2
0.065
0.000135
0
0
0
0
177
0.367
10.8
0.0224
Bromodichloromethane
0
0
0
0
19.5
0.64
0.0174
0.000573
0
0
0
0
35.7
1.17
2.88
0.0949
Bromoform
0
0
96.3
0.065
0
0
177
10.8
Dibromochloromethane
0
0
0
0
4.19
0.187
0.0104
0.000463
0
0
0
0
7.69
0.342
1.73
0.0768
Haloacetic acids
(5HAA’s)
0
0
115
0.287
0
0
210
47.5
Total residual chlorine
276
140
0
0
367
187
0
0
505
257
0
0
672
342
0
0
Total (Membrane
Desalination) (a)
276
140
0
0
698.29
188.027
0.4448
0.001171
505
257
0
0
1279.39
343.879
73.71
0.1941
9-35

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-11. Model Plant Pollutant Loadings for WTPs Performing Chlorination by Source Water Treatment Type and Residuals
Treatment Type (With and Without Dechlorination) for Direct and Indirect (Pass Through) Discharges: Population Served of 10,001 to
100,000 People
Source Water
Treatment Type and
Pollutant
Pollutant Loadings for Model Plants Serving 10,001 to 50,000 People
Pollutant Loadings for Model Plants Serving 50,001 to 100,000 People
With Dechlorination
Without Dechlorination
With Dechlorination
Without Dechlorination
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE (lb-
eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Ion Exchange and Adsorption
Total trihalomethanes
0
0
0.816
0.202
0
0
7.82
0.202
Chloroform
0
0
0
0
18.4
0.0383
4.56
0.00947
0
0
0
0
177
0.367
4.56
0.00947
Bromodichloromethane
0
0
0
0
3.72
0.122
1.22
0.0401
0
0
0
0
35.7
1.17
1.22
0.0401
Bromoform
0
0
18.4
4.56
0
0
177
4.56
Dibromochloromethane
0
0
0
0
0.802
0.0357
0.729
0.0324
0
0
0
0
7.69
0.342
0.729
0.0324
Haloacetic acids
(5HAA’s)
0
0
21.9
20.1
0
0
210
20.1
Total residual chlorine
52.7
26.8
0
0
70.1
35.7
0
0
505
257
0
0
672
342
0
0
Total (Ion Exchange &
Adsorption) (a)
52.7
26.8
0
0
133.322
35.896
31.169
0.08197
505
257
0
0
1279.39
343.879
31.169
0.08197
Source: U.S. EPA, 2008a; U.S. EPA, 2009b.
Blanks indicate that for this pollutant, no TWF is available and therefore, no TWPE were calculated. EPA does not derive TWFs for conventional pollutants.
Zero indicates that EPA estimates the load for this pollutant at zero lbs/yr.
a – Excluded total trihalomethanes from totals to prevent double counting; individual trihalomethane compounds are included in the total.
9-36

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-12. Model Plant Pollutant Loadings for WTPs Performing Chlorination by Source Water Treatment Type and Residuals
Treatment Type (With and Without Dechlorination) for Direct and Indirect (Pass Through) Discharges: Population Served Greater than
100,000 People
Source Water
Treatment Type and
Pollutant
Pollutant Loadings for Model Plants Serving 100,001 to 500,000 People
Pollutant Loadings for Model Plants Serving >500,000 People
With Dechlorination
Without Dechlorination
With Dechlorination
Without Dechlorination
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Precipitative Softening
Total trihalomethanes
0
0
25.8
0.622
0
Not applicable. No
plants in this model
plant group (based on
plant size).
38.6
0.622
Chloroform
0
0
0
0
583
1.21
14.1
0.0292
0
0
872
1.81
14.1
0.0292
Bromodichloromethane
0
0
0
0
118
3.87
3.76
0.124
0
0
176
5.79
3.76
0.124
Bromoform
0
0
583
14.1
0
872
14.1
Dibromochloromethane
0
0
0
0
25.4
1.13
2.25
0.1
0
0
37.9
1.69
2.25
0.1
Haloacetic acids
(5HAA’s)
0
0
694
61.9
0
1040
61.9
Total residual chlorine
1,670
849
0
0
2,220
1,130
0
0
2,490
1,270
3,320
1,690
0
0
Total (Precipitative
Softening) (a)
1,670
849
0
0
4,223
1,136
96
0
2,490
1,270
6,318
1,699
96
0
Coagulation & Filtration
Total trihalomethanes
0
0
8.26
0.507
0
0
23.6
0.534
Chloroform
0
0
0
0
187
0.388
11.5
0.0238
0
0
0
0
532
1.11
12.1
0.0251
Bromodichloromethane
0
0
0
0
37.7
1.24
3.06
0.101
0
0
0
0
107
3.54
3.22
0.106
Bromoform
0
0
187
11.5
0
0
532
12.1
Dibromochloromethane
0
0
0
0
8.13
0.361
1.83
0.0816
0
0
0
0
23.2
1.03
1.93
0.0858
Haloacetic acids
(5HAA’s)
0
0
222
50.5
0
0
633
53.1
Total residual chlorine
534
272
0
0
710
362
0
0
1520
776
0
0
2,030
1,030
0
0
Total (Coagulation &
Filtration) (a)
534
272
0
0
1,352
364
78
0
1,520
776
0
0
3,857
1,036
82
0.22
9-37

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-12. Model Plant Pollutant Loadings for WTPs Performing Chlorination by Source Water Treatment Type and Residuals
Treatment Type (With and Without Dechlorination) for Direct and Indirect (Pass Through) Discharges: Population Served Greater than
100,000 People
Source Water
Treatment Type and
Pollutant
Pollutant Loadings for Model Plants Serving 100,001 to 500,000 People
Pollutant Loadings for Model Plants Serving >500,000 People
With Dechlorination
Without Dechlorination
With Dechlorination
Without Dechlorination
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Direct Discharge
Indirect Discharge
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Filtration Only
Total trihalomethanes
0
0
7.13
0.314
0
Not applicable. No
plants in this model
plant group (based on
plant size).
7.13
0.314
Chloroform
0
0
0
0
161
0.335
7.08
0.0147
0
0
161
0.335
7.08
0.0147
Bromodichloromethane
0
0
0
0
32.5
1.07
1.89
0.0624
0
0
32.5
1.07
1.89
0.0624
Bromoform
0
0
161
7.08
0
161
7.08
Dibromochloromethane
0
0
0
0
7.01
0.312
1.13
0.0504
0
0
7.01
0.312
1.13
0.0504
Haloacetic acids
(5HAA’s)
0
0
192
31.2
0
192
31.2
Total residual chlorine
461
235
0
0
613
312
0
0
461
235
613
312
0
0
Total (Filtration
Only) (a)
461
235
0
0
1166.51
313.717
48.38
0.1275
461
235
1166.51
313.717
48.38
0.1275
Membrane Desalination
Total trihalomethanes
0
0
7.82
1.03
Not applicable. No plants in this model plant group (based on plant size).
Chloroform
0
0
0
0
177
0.367
23.2
0.0482
Bromodichloromethane
0
0
0
0
35.7
1.17
6.21
0.204
Bromoform
0
0
177
23.2
Dibromochloromethane
0
0
0
0
7.69
0.342
3.71
0.165
Haloacetic acids
(5HAA’s)
0
0
210
102
Total residual chlorine
505
257
0
0
672
342
0
0
Total (Membrane
Desalination) (a)
505
257
0
0
1,279
344
158
0
Ion Exchange & Adsorption
Not applicable. No plants in this model plant group (based on plant size).
Source: U.S. EPA, 2008a; U.S. EPA, 2009b.
Blanks indicate that for this pollutant, no TWF is available and therefore, no TWPE were calculated. EPA does not derive TWFs for conventional pollutants.
Zero indicates that EPA estimates the load for this pollutant at zero lbs/yr.
a – Excluded total trihalomethanes from totals to prevent double counting; individual trihalomethane compounds are included in the total.
9-38

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
9.6 NATIONAL POLLUTANT DISCHARGE ESTIMATES
EPA estimated the national discharges of pollutants from WTPs serving more
than 10,000 people (all four size categories) using the model plant loadings presented in Section
9.5 and national estimates of WTP counts (see Appendix E). For WTPs classified as both direct
and indirect dischargers, EPA assumed that half would discharge pollutant loadings similar to
direct dischargers and half would discharge pollutant loadings similar to indirect dischargers
(i.e., pass through the POTW). For example, national estimates list a total of 49 coagulation and
filtration plants, performing dechlorination, and serving between 10,001 and 50,000 people. Of
these 49 plants, 39 are direct dischargers, 2 are indirect discharges, and eight discharge both
directly and indirectly. For the pollutant loadings calculations, EPA used the following WTP
counts:
• 43 direct dischargers (39 direct + 4 both); and
• 6 indirect dischargers (2 indirect + 4 both).
EPA used Equation 9-6 and Equation 9-7 to estimate industry pollutant loadings.
Load
Industry
= Σ (Load
Model Plant
× WTP Count
Model Plant
) (Eq. 9-6)
where:
Load
Industry
= Total industry loadings, in pounds per year (lb/year), for
the model plant group.
Load
Model Plant
= Pollutant loadings, in lb/year, taking into account any
pollutant removals by the POTW for indirect dischargers.
WTP Count
Model Plant
= National estimate of total number of WTPs for the
corresponding model plant group.
TWPE
Industry
= Σ (TWPE
Model Plant
× WTP Count
Model Plant
) (Eq. 9-7)
where:
TWPE
Industry
= Total industry loadings, in toxic weighted pound
equivalents per year (lb-eq/yr), for the model plant group.
TWPE
Model Plant
= Pollutant loadings, in lb-eq/year, taking into account any
pollutant removals by the POTW for indirect dischargers.
WTP Count
Model Plant
= National estimate of total number of WTPs for the
corresponding model plant group.
9-39

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-13 presents a summary of the industry pollutant discharges by source
water treatment type and pollutant, including an estimate of pollutant loadings per facility for
each of the five source water treatment types.
Table 9-14 presents the industry pollutant discharges without pollutant detail by
source water treatment type and WTP size category. The total discharges from the industry are
352 million pounds per year (excluding bulk parameters to prevent double counting of pollutant
loadings) and 415,000 toxic-weighted pound equivalents (TWPE) per year. Most of the TWPE
(85 percent) is due to five pollutants:
1. Total Residual Chlorine: 120,000 lb-eq/yr;
2. Aluminum: 88,600 lb-eq/yr;
3. Copper: 60,700 lb-eq/yr;
4. Manganese: 41,800 lb-eq/yr; and
5. Fluoride: 41,100 lb-eq/yr.
Discharges of Chloramines
As discussed in Section 8, total residual chlorine (TRC) is the amount of chlorine
remaining in the wastewater after chlorine demand. TRC is the summation of free chlorine and
combined chlorine (chloramines). The industry discharges of TRC total 235,000 pounds per year
and 120,000 toxic-weighted pound equivalents per year. EPA does not have data available to
determine the portion of TRC that is chloramines versus free chlorine. Therefore, EPA cannot
estimate the percent of TRC loadings attributed to chloramines. EPA did collect data in the
industry questionnaire to estimate the number of WTPs using chloramines for primary
disinfection. From national estimates, 318 of 2,002 WTPs performing primary disinfection and
serving more than 10,000 people use chloramines as their primary disinfectant, or approximately
16 percent of plants that perform primary disinfection. Most of the plants (192 of 318 WTPs)
serve less than 50,000 people. For the larger plants
• 83 are precipitative softening plants;
• 37 are conventional filtration plants; and
• 6 are membrane desalination, microfiltration, or ultrafiltration plants.
9-40

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-13. Pollutant Loadings
a
for WTPs: National Estimates by Source Water Treatment Type and Pollutant
Pollutants
Precipitative Softening
Coagulation and
Filtration
Filtration Only
Membrane Desalination
Ion Exchange and
Adsorption
INDUSTRY TOTAL
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Bulk Parameters
BOD
1,160,000
885,000
31,200
2,070,000
CBOD
31,000
9,610
40,600
Nitrogen, Total
3,020,000
2,460,000
90,000
97,800
12,300
5,680,000
TDS
252,000,000
252,000,000
TSS
275,000,000
38,700,000
65,800
88,500
60,500
314,000,000
Total Bulk
Parameters
280,000,000
42,100,000
187,000
217,000
252,000,000
574,000,000
Specific Parameters
Aluminum
142,000
9,130
1,320,000
78,200
19,700
1,270
1,480,000
88,600
Ammonia
408,000
453
344,000
356
12,800
14.2
53,300
59.2
1,950
2
820,000
884
Barium
8,330
16.5
6,810
12.6
15,100
29.1
Cadmium
32
740
9.7
224
41.8
964
Calcium
7,630,000
213
6,760,000
177
14,400,000
390
Chlorides
236,000,000
5,740
90,500,000
2,210
326,000,000
7,950
Copper
55,800
35,400
43,100
25,300
27.9
17.7
16.1
10.2
99,000
60,700
Fluoride
642,000
22,500
457,000
14,900
4,459
156
69,300
2,430
34,600
1,210
1,210,000
41,100
Iron
92,800
519
1,830,000
9,490
2,860
16
46,100
259
4,100
23
1,970,000
10,300
Lead
4,610
10,300
3,620
7,500
129
290
0
0
8,360
18,100
Magnesium
2,240,000
1,940
1,960,000
1,590
4,200,000
3,530
Manganese
292,000
20,600
313,000
20,600
1,511
106
7,880
556
615,000
41,800
Nickel
0
0
0
0
0
0
Phosphorus, Total
346,000
277,000
9,981
2,186
1,400
637,000
0
Zinc
256,000
12,000
200,000
8,660
57
2.67
456,000
20,600
Total Specific
Parameters
12,100,000
113,000
13,500,000
167,000
51,400
1,860
236,000,000
9,240
90,600,000
4,240
352,000,000
295,000
9-41

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-13. Pollutant Loadings
a
for WTPs: National Estimates by Source Water Treatment Type and Pollutant
Pollutants
Precipitative Softening
Coagulation and
Filtration
Filtration Only
Membrane Desalination
Ion Exchange and
Adsorption
INDUSTRY TOTAL
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Lb/yr
TWPE
(lb-eq/yr)
Pollutants from Chlorination
Total
trihalomethanes
1,510
0
830
0
27.5
0
12.8
0
11.5
0
2,390
0
Chloroform
34,000
70.7
18,800
39
621
1.29
290
0.601
260
0.54
54,000
112
Bromodichloromet
hane
6,950
228
3,950
130
132
4.34
58.7
1.93
69.5
2.29
11,200
367
Bromoform
34,000
0
18,800
0
621
0
290
0
260
0
54,000
0
Dibromochloromet
hane
1,610
71.4
1,090
48.7
38.5
1.72
12.7
0.566
41.6
1.85
2,790
124
Haloacetic acids
(5HAA’s)
43,900
0
30,000
0
1,050
0
348
0
1,150
0
76,400
0
Total residual
chlorine
139,000
70,600
91,600
46,700
2,620
1,330
1,100
561
1,000
509
235,000
120,000
Total From
Chlorination (b)
259,000
71,000
164,000
46,900
5,090
1,340
2,100
564
2,780
514
433,000
120,000
Total Specific
Pollutants plus
Chlorination
Pollutants
12,400,000
184,000
13,700,000
214,000
56,500
3,200
236,000,000
9,800
90,600,000
4,750
352,000,000
415,000
Number of WTPs
(a)
349
1,010
97
41
92
1,620
Loads per WTP --
Bulk Parameters
801,000
41,800
1,930
5,300
2,740,000
354,000
Loads per WTP) --
Specific Pollutants
34,700
324
13,400
166
530
19.1
5,760,000
225
984,000
46.1
217,000
182
Loads per WTP --
Chlorination
Pollutants
743
203
163
46.7
52.5
13.8
51.2
13.8
30.2
5.59
267
74.1
Loads per WTP –
Specific Pollutants
plus Chlorination
Pollutants
35,400
527
13,600
213
583
32.9
5,760,000
239
984,000
51.7
217,000
256
Source: U.S. EPA, 2009b.
a – Loadings include only those pollutants included in the analysis (see Section 9.3).
b – Excluded total trihalomethanes from totals to prevent double counting; individual trihalomethane compounds are included in the total.
9-42

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
Table 9-14. Pollutant Loadings
a
for WTPs Serving More than 10,000 People: National
Estimate by Source Water Treatment Type and WTP Size (as Population Served)
Source Water
Treatment Type
WTPs Serving 10,001 to
50,000 People
WTPs Serving 50,001
to 100,000 People
WTPs Serving
100,001 to 500,000
People
WTPs Serving
>500,000 People
Total
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-
eq/yr)
Lb/yr
TWPE
(lb-
eq/yr)
Precipitative
Softening
1,380,000
16,700
1,040,000
13,400
7,950,000
124,000
2,000,000
30,300
12,400,000 lb/yr
(184,000 lb-eq/yr)
Coagulation and
Filtration
5,150,000
66,500
2,250,000
37,300
5,020,000
87,600
1,250,000
22,200
13,700,000 lb/yr
(214,000 lb-eq/yr)
Filtration Only
41,000
2,630
663
14.1
14,000
536
760
14.3
56,500 lb/yr
(3,200 lb-eq/yr)
Membrane
Desalination
191,000,000
8,100
4,800,000
170
40,400,000
1,540
0
0
236,000,000 lb/yr
(9,800 lb-eq/yr)
Ion Exchange and
Adsorption
86,000,000
4,520
2,020,000
80.8
2,500,000
155
0
0
90,600,000 lb/yr
(4,800 lb-eq/yr)
Total by WTP Size
283,000,000
98,400
10,100,000
50,900
55,900,000
214,000
3,250,000
52,500
352,000,000 lb/yr
(415,000 lb-
eq/yr)
Source: U.S. EPA, 2009b.
a – Loadings include only those pollutants included in the analysis. Totals exclude total trihalomethanes (individual trihalomethanes are included
in the total) and bulk parameters to prevent double counting of pollutant loadings.
9.7 REFERENCES
Eastern Research Group (ERG), 2009. Memorandum: Pollutant Loadings Estimates for Drinking
Water Treatment Plants: Model Plants and National Estimates, January 29, 2009. Chantilly, VA.
DCN DW03777.
Malmrose, et al., 2004. Paul Malmrose, Jim Lozier, Michael Mickley, Robert Reiss, Jerry
Russell, James Schaefer, Sandeep Sethi, Jennifer Manuszak, Robert Bergman, and Khalil Z.
Atasi, Residual Management Research Committee Subcommittee on Membrane Residual
Management, “2004 Committee Report: Residuals Management for Desalting Membranes,”
Jour. AWWA, 96:12:73. American Water Works Association (AWWA), December 2004.
Document Control Number (DCN) DW00032.
U.S. Environmental Protection Agency (U.S. EPA), 1982. Fate of Priority Pollutants in Publicly
Owned Treatment Works (EPA 440/1-82/303, September 1982.
U.S. EPA, 1994. National Risk Management Research Laboratory (NRMRL) Treatability
Database Version 5.0, Cincinnati, OH.
U.S. EPA. 2006. Toxic Weighting Factor Development in Support of CWA 304(m) Planning
Process. Washington, DC. (June). EPA-HQ-OW-2004-0032-1634.
U.S. EPA, 2007. Phase I Discharge Monitoring Report (DMR) Database, Office of Water,
Washington, DC. DCN DW03703.
9-43

Drinking Water Industry Report Section 9 – Water Treatment Plant Pollutant Discharge Estimates
U.S. EPA, 2008a. Drinking Water 2006 Baseline Pollutant Loadings Database, Office of Water,
Washington, DC. DCN DW03741
U.S. EPA, 2008b. National Primary Drinking Water Standards (List of Drinking Water
Contaminants and MCLs) (original document published in 2003, DCN DW00657), Office of
Ground Water and Drinking Water, Washington, DC. Retrieved from
http://www.epa.gov/safewater/contaminants/index.html Accessed April 2008 for updates.
U.S. EPA, 2009a. Drinking Water Survey Response Database – Technical Data for the 2006
Drinking Water Industry Questionnaire. Office of Water, Washington, DC. DCN DW03790.
U.S. EPA, 2009b. Drinking Water Treatment Industry Review: Estimated Discharge of
Pollutants from Water Treatment Plants (WTPs) Serving More than 10,000 People(MS Excel
spreadsheet), Office of Water, Washington, DC. DCN DW03776
U.S. EPA, ASCE, and AWWA, 1996. Technology Transfer Handbook: Management of Water
Treatment Plant Residuals (EPA 625-R-95-008), Office of Research and Development,
Washington, DC. DCN DW03736.
9-44

SECTION 10
POTENTIAL SCOPE OF ENVIRONMENTAL IMPACTS OF
POLLUTANT DISCHARGES
As part of its review of the drinking water treatment industry, EPA assessed the
potential scope of environmental impacts from surface water discharges of water treatment plant
(WTP) residuals. The purpose of the assessment was to better understand, at the national level,
the degree to which discharges of residuals may be causing environmental harm.
Due to incomplete data, EPA is unable to draw conclusions about the extent and
magnitude of potential environmental impacts from WTP discharges. EPA did not conduct
sampling of WTP discharges, so the analysis of environmental impacts typically performed for
an effluent guidelines rulemaking was not performed. Instead, EPA reviewed publicly available
information about potential environmental impacts.
10.1 REVIEW OF PUBLICLY AVAILABLE INFORMATION
EPA reviewed major on-line research services, together with a search of the
websites of 18 drinking water treatment utilities and industry organizations. The search yielded
197 references and EPA reviewed 106 articles published between 1984 and 2005, including
articles from U.S. regional newspapers and trade journals. The articles identified only a few
environmental impact issues associated with WTP discharges. The majority of articles (26
articles) concern the disposal of desalination concentrate, particularly in Tampa Bay, Florida,
and discharges from the Washington Aqueduct WTP to the Potomac River in Washington, DC.
Other articles about specific plants include reporting of a treatment chemical spill in North
Carolina, an unpermitted WTP discharge in Massachusetts, alum discharge issues at a WTP in
Arkansas, and a permit application in Virginia involving desalination concentrate discharges.
Key points about potential environmental impacts found in studies and journal
articles include:
10-1

Drinking Water Industry Report Section 10 – Potential Environmental Impacts
• Alum and lime sludge discharges pose a threat to aquatic life through
benthic smothering downstream of outfalls.
• Aluminum and other metals present in alum sludge can be toxic to aquatic
organisms (AWWARF, 1987; George, 1995; Sotero-Santos et al., 2005;
Tumeo, 1992).
• WTPs that accumulate sludge in settling basins for several months and
then discharge in batches periodically, increase the magnitude of potential
environmental impacts.
• The flow and volume of the receiving waterbody is a factor in the degree
of impacts from alum and lime sludge. If the flow is low, then sludge will
more readily fall out of suspension in the water column and coat the
bottom (U.S. EPA/ASCE/AWWA, 1996).
10.2 SUMMARY OF ENVIRONMENTAL IMPACT OF WTP RESIDUALS BY
POLLUTANT
This section provides details on the pollutants highlighted in the review of readily
available information for the environmental impacts and common pollutants found in WTP
residuals (see Section 8). The information in this section is not specific to WTP discharges.
10.2.1 Environmental Impact of Solids
Suspended solids discharged by WTPs may settle to form bottom deposits in the
receiving water, creating anaerobic conditions because of the oxygen demand exerted by
microbial decomposition. Suspended solids also increase turbidity in receiving waters and reduce
light penetration through the water column, thereby limiting the growth of rooted aquatic
vegetation that serves as a critical habitat for fish, shellfish, and other aquatic organisms.
Suspended solids also provide a medium for the transport of other sorbed pollutants, including
nutrients, pathogens, metals, and toxic organic compounds, which accumulate in settled deposits.
Settled suspended solids and other associated pollutants often have extended interaction with the
water column through cycles of deposition, resuspension, and redeposition.
10-2

Drinking Water Industry Report Section 10 – Potential Environmental Impacts
In addition, suspended solids in wastewater discharges can clog fish gills. In
severe situations, clogging of fish gills can result in asphyxiation; in less severe situations, it can
result in an increase in susceptibility to infection.
Dissolved solids can have a potential impact on the subsequent use of receiving
waters that serve as source waters for public and industrial water supplies. Dissolved solids also
have the potential to alter the chemistry of natural waters to a degree that adversely affects
indigenous aquatic biota, especially in the immediate vicinity of the effluent discharge. An
example is a possible influence on the toxicity of heavy metals and organic compounds to fish
and other aquatic organisms, primarily because of the antagonistic effect of hardness.
10.2.2 Environmental Impact of Metals
Metals are potentially toxic to phytoplankton and zooplankton and to higher
aquatic plant and animal species, including fish. They also have the potential for
bioaccumulation and biomagnification in aquatic food chains and presence downstream in
effluent receiving waters used as source waters for potable water supplies.
Aluminum is toxic in the aquatic environment. The direct effect of WTP residuals
on the aquatic environment is difficult to isolate from the effect of naturally-occurring aluminum.
The aluminum species concentration causing toxicity depends on water chemistry, aquatic
organism affected, and the effect being monitored. Studies on the toxic effects of aluminum in
the aquatic environment have shown that inorganic aluminum can be toxic to several fresh-water
species of fish, invertebrates, bacteria, and algae at pH conditions less than 6 (U.S. EPA, ASCE,
AWWA, 1996).
10.2.3 Environmental Impact of Chlorine and Chloramines
WTPs commonly use chlorine and chloramines to disinfect drinking water. These
chemicals may become part of residuals waste streams either by addition prior to residuals
generation (primary disinfection) or by using finished drinking water as backwash (disinfection
at the clear well is secondary disinfection).
10-3

Drinking Water Industry Report Section 10 – Potential Environmental Impacts
Free chlorine is directly toxic to aquatic organisms and can react with naturally
occurring organic compounds in receiving waters to form toxic compounds such as
trihalomethane. Chloramines can remain chemically stable in water from hours to days. They are
highly toxic to fish and other organisms which live in water. These substances are not found to
be bioaccumulative, or to transfer up the food chain (Environment Canada, 2002).
10.2.4 Environmental Impact of Oxygen Demand
When WTP wastewaters are discharged to surface waters, the microorganisms
present in the naturally occurring microbial ecosystem decompose the organic matter contained
in the wastewater. The decomposition process consumes oxygen and reduces the amount
available for aquatic animals. Severe reductions in dissolved oxygen concentrations can lead to
fish kills. Even moderate decreases in dissolved oxygen concentrations can adversely affect
waterbodies through decreases in biodiversity, as manifested by the loss of some species of fish
and other aquatic animals. Loss of biodiversity in aquatic plant communities due to anoxic (i.e.,
insufficient oxygen) conditions can also occur.
10.2.5 Environmental Impact of Chlorides
Chloride (Cl-) is a common anion in wastewaters and natural waters. For the
protection of freshwater fish and aquatic life, EPA recommends the following for chloride:
criteria maximum concentration of 860 mg/L (acute effects) and criterion continuous
concentration of 230 mg/L (chronic effects) (U.S. EPA, 2006). Exceeding these chloride levels
in wastewater discharges can be harmful to animals and plants in non-marine surface waters and
can disrupt ecosystem structure. It can also adversely affect biological wastewater treatment
processes. Furthermore, excessively high chloride concentrations in surface waters can impair
their use as source waters for potable water supplies. If sodium is the predominant cation present,
the water will have an unpleasant taste due to the corrosive action of chloride ions.
10-4

Drinking Water Industry Report Section 10 – Potential Environmental Impacts
10.2.6 Environmental Impact of Nitrogen
Under both anaerobic and aerobic conditions, the readily biodegradable fraction
of organic nitrogen is mineralized readily by microbial activity. The nitrogen not used for cell
synthesis accumulates as ammonia nitrogen. The water quality impacts associated with organic
nitrogen are related to this process of mineralization to ammonia nitrogen in natural waters and
are discussed below.
Both ammonia nitrogen and ammonium nitrogen can be directly toxic to fish and
other aquatic organisms; ammonia nitrogen is the more toxic. In addition, discharges of ammonia
nitrogen can reduce ambient dissolved oxygen concentrations in receiving surface waters
because of the microbially mediated oxidation of ammonia nitrogen to nitrite plus nitrate
nitrogen. This demand is known as nitrogenous oxygen demand (NOD).
Ammonia nitrogen in wastewater discharges can also be responsible for the
development of eutrophic conditions in the receiving water. Eutrophic waters are rich in mineral
and organic nutrients. These nutrients promote the growth of plant life, especially algae. Plants
reduce the dissolved oxygen content. These adverse impacts on ambient dissolved oxygen
concentrations occur if nitrogen is the nutrient limiting primary productivity. Although
phosphorus is typically the nutrient limiting primary productivity in fresh surface waters,
nitrogen is typically the limiting nutrient in marine waters and the more saline segments of
estuaries. Algae blooms from eutrophic conditions cause shifts in ambient dissolved oxygen
concentrations from supersaturation on sunny days to substantial deficits at night and on cloudy
days, when photosynthesis does not occur. The decay of the biomass generated by excessive
primary productivity also exerts a demand on ambient dissolved oxygen concentrations. With the
depression of ambient dissolved oxygen concentrations, populations of fish and other aquatic
organisms are adversely affected, possibly causing a change in ecosystem composition and a loss
of biodiversity.
Although nitrite plus nitrate nitrogen exerts an NOD in surface waters, the
principal concern about oxidized forms of nitrogen in wastewater discharges is related to their
role in the development of eutrophic conditions. The impacts of such conditions on fish
10-5

Drinking Water Industry Report Section 10 – Potential Environmental Impacts
populations, biodiversity, recreation, and potable water supply are discussed above. An
additional concern is their potential for increasing ambient surface water nitrate (as nitrogen) and
nitrite (as nitrogen) concentrations above the national maximum contaminant levels in source
waters used for public drinking water supplies.
10.2.7 Environmental Impact of pH Changes
The hydrogen-ion concentration in an aqueous solution is represented by the pH,
which is defined as the negative logarithm of the hydrogen-ion concentration in a solution. On
the pH scale ranging from zero to 14, a value of seven represents neutral conditions—the
concentrations of hydrogen (H+) and hydroxyl ions (OH-) are equal. pH values less than seven
indicate acidic conditions and values greater than seven represent basic conditions.
WTPs adjust the pH to optimize source water treatment, and the addition of lime
for softening raises the pH of the water. pH varies in WTP wastewaters and can have negative
impacts on receiving water. Wastewaters with pH values markedly different from the receiving
stream pH can have a detrimental effect on the environment. Sudden pH changes can kill aquatic
life.
10.2.8 Environmental Impact of Phosphorus
Phosphorus is the nutrient typically limiting primary productivity in freshwater
ecosystems. In such aquatic ecosystems, an increase in ambient phosphorus concentration due to
wastewater discharges above naturally occurring levels results in the excessive growth of algae
and other phytoplankton, with the development of eutrophic conditions as the consequence. In
turn, eutrophic conditions can cause fish kills, disruption of natural aquatic ecosystem structure,
and loss of biodiversity. In marine waters, phosphorus is not as much of a concern because of
relatively high naturally occurring phosphorus concentrations. The impact of phosphorus in
wastewater discharges into estuaries varies—in general, impacts decrease as salinity levels
increase.
10-6

Drinking Water Industry Report Section 10 – Potential Environmental Impacts
10.2.9 Environmental Impact of Radionuclides
Radionuclides regulated in drinking water include combined radium -226/-228,
(adjusted) gross alpha, beta particle and photon radioactivity, and uranium. Exposure to
radionuclides from drinking water results in the increased risk of cancer. Exposure to elevated
uranium levels in drinking water has been shown to lead to changes in kidney function that are
indicators of potential future kidney failure (U.S. EPA, 2000).
10.3 REFERENCES
American Public Health Association (APHA), 1995. Standard Methods for the Examination of
Water and Wastewater, 19
th
edition, Washington, DC.
American Water Works Association Research Foundation (AWWARF), 1987. Water Treatment
Plant Waste Management. Prepared by Environmental Engineering and Technology, Newport
News, VA for AWWARF, Denver, CO. Document Control Number (DCN) DW00186.
Eastern Research Group (ERG), 2006. Memorandum: Literature Review Results for the Drinking
Water Environmental Assessment and Summary of the Environmental Impacts of Water
Treatment Residuals Discharged to Surface Waters (April 28, 2006). Chantilly, VA. DCN
DW00626.
Environment Canada, 2002. Backgrounder: Chloramines, Canadian Environmental Protection
Act (CEPA) Environmental Registry, Retrieved from
http://www.ec.gc.ca/CEPARegistry/subs_list/Chloramines_BG.cfm, last updated September 13,
2002. DCN DW00423.
George, 1995. Dennis B. George, et al. Alum Sludge in the Aquatic Environment [Project #319].
AWWARF, Denver, CO. DCN DW00449.
Sotero-Santos, Rosana B., Odete Rocha, and Jurandyr Povinelli. 2005. Evaluation of water
treatment sludges toxicity using the Daphnia bioassay. Water Research 39: 3909-3917. DCN
DW00530.
Sutherland, David, W. 1999. Washington Aqueduct Sediment Discharges: Report of Panel
Recommendations. Chesapeake Bay Field Office. U.S. Fish and Wildlife Office. DCN DW00534
Tumeo, Mark A, 1992. Effects of Lime-Sludge Discharge on an Arctic River [Paper Number
92102]. American Water Resources Association. Issue Volume 28, Number 6, December 1992,
pages 1083-1094.
10-7

Drinking Water Industry Report Section 10 – Potential Environmental Impacts
U.S. EPA, 2000. Technical Fact Sheet: Final Rule for (Non-Radon) Radionuclides in Drinking
Water (EPA 815-F-00-013), Office of Ground Water and Drinking Water, Washington, DC.
Retrieved from http://www.epa.gov/safewater/radionuclides/regulation_techfactsheet.html.
U.S. EPA, 2006. National Recommended Water Quality Criteria, Office of Water, Washington,
DC. DCN DW01149.
U.S. EPA, ASCE, and AWWA, 1996. Technology Transfer Handbook: Management of Water
Treatment Plant Residuals (EPA 625-R-95-008), Office of Research and Development,
Washington, DC. DCN DW03736.
10-8

SECTION 11
TECHNOLOGIES AND PRACTICES FOR PREVENTING,
TREATING, DISPOSING OF, AND DISCHARGING SOURCE
WATER TREATMENT RESIDUALS
Water treatment plants (WTPs) use control technologies and management
practices to improve the prevention, treatment, disposal, and discharge of source water treatment
residuals. Adoption of certain control technologies and management practices may significantly
help WTPs meet permit limits. Other benefits of control technologies and management practices
include improved water quality, reduced treatment system operation costs, avoidance of NPDES
permitting costs, and energy savings.
The Clean Water Act (CWA) authorizes EPA to require WTPs to implement best
management practices (BMPs) as part of their National Pollutant Discharge Elimination System
(NPDES) permits. EPA has the flexibility to include BMPs in addition to pollutant concentration
limits or in lieu of pollutant limits. Examples of BMPs in permits include establishing schedules
of activities; prohibitions of practices; maintenance procedures; treatment requirements; and
operating procedures and practices to control plant site runoff, leaks and spills, sludge or waste
disposal, and drainage from raw material storage areas.
When applied to WTPs, the Pollution Prevention Act of 1990 and EPA’s national
pollution prevention policy
24
, provide a framework for determining BMPs, beginning with
pollution prevention at the source, followed by recycling of filter backwash, efficient treatment
of residuals, land disposal of solids and certain waste streams, and practices to minimize the
potential aquatic impacts of the discharge of residuals. This chapter discusses a range of BMPs,
organized according to their placement in the hierarchy:
• Pollution prevention and waste reduction (Section 11.1);
• Residuals treatment (Section 11.2);
24
See http://www.epa.gov/p2/pubs/p2policy/definitions.htm#national for a description of EPA’s national pollution
prevention policy.
11-1

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
• Disposal of wastes (Section 11.3); and
• Discharge of wastes (Section 11.4).
WTPs that do not discharge treatment residuals to surface water or to POTWs are
not required to obtain a NPDES permit, and thus exemplify the most effective application of
BMPs. Zero discharging WTPs that generate residuals but do not discharge, are not required to
obtain a NPDES permit. Becoming a zero discharging WTP results in multiple benefits such as
water conservation, environmental improvements, and cost reduction. Most plants achieve zero
discharge status through a combination of pollution prevention/waste management and residuals
treatment practices, such as recycling, evaporation, composting, landfill disposal, spray
irrigation, underground injection, and land application. EPA’s 2006 survey found that 70 percent
of WTPs perform one or more of these methods to reduce discharges to surface waters or
POTWs and 25 percent have achieved zero discharge status (see Appendix A).
Ground water plants use primarily underground injection control, recycling, and
landfill disposal to achieve zero discharge. Surface water plants use recycling, landfill disposal,
and land application (see Appendix A).
11.1 POLLUTION PREVENTION AND WASTE REDUCTION
This section discusses pollution prevention (e.g., process modifications) and
waste reduction (e.g., resource recovery) opportunities at WTPs to reduce the generation of
residuals during source water treatment.
25
Pollution prevention and waste reduction practices
may also benefit WTPs by reducing operating costs, reducing risk of liability, and improving
system or plant image, without compromising the finished water quality.
As part of the 2006 industry survey, EPA collected data on pollution prevention
and waste reduction practices at WTPs. Figure 11-1 presents the distribution of pollution
prevention and waste reduction practices commonly found at WTPs serving more than 10,000
people.
25
Pollution prevention is the use of materials, processes, or practices that reduce or eliminate the creation of
pollutants or waste at the source (U.S. EPA, 1992).
11-2

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
TOTAL
Optimizing
source water
intake
Recycling of
filter
backwash
Recycling of
filter-to-
waste
Recovery of
treatment
chemicals
Recycle of
softening
chemicals
Other
No pollution
prevention
0
200
400
600
800
1000
1200
1400
1600
Number of WTPs
Ground Water Plants (637 WTPs)
Surface Water Plants (1,514 WTPs)
Figure 11-1. WTP Pollution Prevention and Waste Reduction Practices in the U.S. in 2006
Source: Appendix A.
WTP pollution prevention and waste reduction options discussed in this section
include:
• Optimizing source water intake conditions to reduce suspended solids and
thereby reduce source water treatment requirements.
• Optimizing filter media for finished water and residuals.
• Optimizing pH to reduce coagulant chemicals used.
• Reducing softening chemicals used by frequent monitoring of source
water hardness.
• Returning backwash water and filter-to-waste to the head of the source
water treatment plant for reuse.
11-3

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
• Reusing precipitative softening chemicals by recycling softening residuals.
• Recovering treatment chemicals.
11.1.1 Optimize Intake Water Conditions
When properly designed, situated, and instrumented, intake structures can play an
important role in regulating the quality, volume, and composition of the source water presented
for treatment. Intake features must be flexible to meet the current and future demands, yet be
durable enough to withstand the rigors of time and nature. Careful placement of the intake
structure (particularly in lakes or reservoirs) allows the WTP to draw water that has lower levels
of total suspended solids, which in turn requires less coagulant to be added and generates a
smaller volume of solid residuals.
An example of a facility that is currently optimizing intake water conditions is the
James J. Corbalis Water Treatment Plant in Fairfax, Virginia. Fairfax Water constructed an
extension that moved the intake structure away from the edge of the river. The new intake
location improved the quality of the source water by decreasing turbidity and total organic
carbon levels, and provided a more consistent day-to-day source that is less influenced by local
runoff. The new intake location resulted in approximately 30 percent lower consumption of
treatment chemicals and a corresponding reduction in residuals generation (U.S. EPA, 2005).
The relocation of the intake pipe in the example above might be an option for
WTPs with high total suspended solids (TSS) and turbidity levels in the source water. While
relocating the intake pipe requires an investment of capital, plants might be able to recoup these
costs over a reasonably cost-effective time frame through savings on operation and maintenance.
11.1.2 Optimize Filter Media
By optimizing filter media, WTPs might be able to maintain or improve finished
water quality, while reducing the quantity of backwash residuals. For example, in 1996, the
Philadelphia Suburban Water Company replaced the support gravel and media in four of the dual
media filters at the Pickering West Water Treatment Plant in Phoenixville, Pennsylvania. The
11-4

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
Philadelphia Suburban Water Company replaced the support gravel and sand with the same size
and quality media in all of the filters. The water company also replaced the anthracite with the
same effective size and quality in all of the filters, but with different uniformity coefficients
(UC)
26
(one at 1.6 UC, one at 1.5 UC, one at 1.4 UC, and one at 1.3 UC). Data gathered over a
one-year period indicated substantial differences in the filter run times and water quality. The
lower anthracite uniformity coefficient showed the following benefits:
• Longer filter run times: up to 50 percent longer;
• Fewer backwashes—up to 33 percent less;
• Increased drinking water production—2 percent higher; and
• Improved water quality—up to 38 percent lower 2-5 micron particle
counts (Cryptosporidium falls into this particle size range) (Yohe, 2006).
Reducing the volume of backwash water residuals can reduce the costs associated with residuals
management, as long as finished water quality is not compromised.
11.1.3 Optimize pH to Reduce Coagulant Chemicals
As water progresses through the source water treatment train at coagulation and
filtration plants, operators add coagulants to enhance the efficiency of solids removal. The
majority of coagulant chemicals settle, along with the removed contaminants, during source
water treatment. The coagulant chemicals then become part of the residuals waste stream. When
selecting a coagulant chemical, plants might consider waste generation along with their finished
water quality goals.
Coagulants contain active ingredients (e.g., aluminum, iron) and impurities (e.g.,
chromium, mercury, nickel, zinc). By reducing the amount of coagulant needed to achieve solids
removal, WTPs also reduce the amount of these coagulant chemicals in the residuals. To
minimize the use of coagulants, WTPs can optimize solids settling using the pH in clarifiers and
sedimentation tanks. The pH of the water affects the performance of alum and ferric coagulation
26
Measure of the particle size variations (ratio).
11-5

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
salts. Alum has a minimum solubility at pH 6, while ferric salts have a minimum solubility at pH
8 (Tchobanoglous, et al., 2003). Thus, the continuous adjusting of pH to keep optimal
coagulation conditions might help to reduce waste products but still effectively treat the source
water.
11.1.4 Reduce Softening Chemicals by Monitoring Source Water Hardness
Similar to coagulation, softening operations add chemicals to adjust the pH, adjust
the bicarbonate equilibrium, and precipitate the calcium hardness as calcium carbonate. WTPs
remove calcium hardness to a level that meets the aesthetic requirements of the customer. By
monitoring the calcium content of the influent, WTPs might reduce the amount of chemicals
needed to precipitate the required fraction of calcium hardness, thus resulting in a minimized
amount of residuals requiring additional treatment or disposal.
11.1.5 Return Backwash Water and Filter-to-Waste to the Head of the Source
Water Treatment Plant for Reuse
Filter backwash water and filter-to-waste are good examples of residuals suitable
for reuse, provided finished drinking water quality is maintained. Usually, finished drinking
water is used as the filter scouring agent to backwash (or clean) the filter. Filter-to-waste is the
initial permeate production when a filter is brought back online following backwashing, and is
part of the backwash waste stream.
The backwash process generates a significant volume of wastewater that can
amount to 2 to 5 percent of plant capacity (U.S. EPA, ASCE, and AWWA, 1996). If allowed to
settle for 24 hours, the majority of the suspended solids in the backwash separate and the effluent
can be returned to the head of the treatment plant for reuse while the solids are managed as
waste. This practice also helps WTPs supplement available source water, which might prove
especially valuable during water shortages. WTPs can also use this approach for decanted
effluents from sludge thickeners and other dewatering liquids, thereby reducing the amount of
effluent discharged.
11-6

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
In 2001, EPA’s Filter Backwash Recycle Rule (FBRR) established requirements
to ensure that WTPs do not compromise the quality of finished drinking water when reusing
water in this manner. The FBRR applies to WTPs that use surface water or ground water under
the direct influence of surface water and operate conventional or direct filtration plants (i.e.,
perform coagulation, filtration, and possibly sedimentation of the intake water). The FBRR
requires WTPs that reuse certain wastewater residuals (i.e., filter backwash, thickener
supernatant, and dewatering process liquids) to return the water to a point in the source water
treatment process where it will be treated by coagulation and filtration. Introduction of reused
waters at any other location requires prior state approval.
The purpose of the FBRR is to reduce the risk of illness from microbial pathogens
in drinking water. During reuse, contaminants might be reintroduced into the source water
treatment plant. The introduction of the contaminants can impair treatment process performance
if not done properly. This can result in contaminants passing through source water treatment and
into the drinking water.
Depending on the source water quality and wastewater characteristics (i.e.,
contaminant levels), some plants might not be able to reuse water streams. For example,
concentrate residuals from membrane systems can concentrate contaminants more than five
times their original concentration in the source water. Returning concentrate to the head of the
treatment plant without extensive pretreatment would put a significant strain on the efficiency of
the membrane and reduce its effectiveness. If the concentrate volume is low, discharge to a
sanitary sewer might be the more affordable alternative to pretreatment and reuse.
11.1.6 Reuse of Precipitative Softening Chemicals
WTPs might reuse precipitative softening chemicals (i.e., lime) to save costs on
purchasing lime and disposing of softening residuals. Lime recovery from the residuals is
accomplished using recalcination, in which the calcium carbonate in the lime softening sludge is
converted to calcium oxide. WTPs perform dewatering and oxidation to complete the
conversion. WTPs generally use centrifugal separators to dewater the calcium carbonate. The
11-7

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
calcium carbonate is then dried and oxidized, usually in a furnace. The recovered lime is
returned back to the source water treatment plant (U.S. EPA, ASCE, and AWWA, 1996).
11.1.7 Recovery of Treatment Chemicals
In addition to the lime recovery discussed above, WTPs might recover coagulants
for reuse at the plant. Assuming recovered treatment chemicals meet purity standards, this
process results in cost savings from reduced cost to dispose of solid waste residuals and reduced
cost for purchasing new treatment chemicals. A second treatment recovery option available to
WTPs is to recover salts from ion exchange concentrate residuals. The salt is a saleable resource.
11.1.7.1 Coagulant Recovery
Most coagulants are cationic (positively-charged) in nature and include the
following chemicals: aluminum (alum), iron (ferric) salts, and a wide variety of organic
polymers. The type and character of the source water, as well as plant choice, determines which
chemicals are used and the degree of possible reuse.
Solubility diagrams for aluminum and iron show that both metals approach their
minimum solubility in the pH range of 6 to 8 (Tchobanoglous, et al., 2003). Because this is the
normal operating range for most utilities, nearly all of the insoluble coagulant components added
are expected to be incorporated in the precipitated solids and to be available for recovery.
Solubility diagrams also show that the solubility for both metals increases as the pH is made
more acidic (less than 6).
The traditional approach for alum recovery has been acid extraction to convert the
alum to a dissolved form for decanting and recycling. Aluminum recovery rates of 60 to 80
percent have been reported at the pH 3 level (ASCE, 1997). However, this approach can also
carry over “native” metals in the source water and the recycled coagulant might be of lesser
purity than American National Standards Institute (ANSI)/National Science Foundation (NSF)
Standard 60. Ion exchange has also been successfully used to recover dissolved aluminum from
acid extraction. Iron recovery can be accomplished in a way that is similar to alum recovery.
11-8

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
Acid extraction at a pH between 1.5 and 2 has produced iron recoveries at 60 to 70 percent
(ASCE, 1997), but dewatering difficulties with the sludge have limited its commercial
application.
Since aluminum and iron are amphoteric (i.e., exhibiting properties of both an
acid and a base), a strong base can also dissolve the metal hydroxides. Treatment with sodium
hydroxide produces a sodium aluminate compound that can be reused as a coagulant in water
treatment. To date, few WTPs recover coagulants due to purity concerns and the low cost
(market price)
27
of purchasing of new chemicals.
11.1.7.2 Salt Recovery Via Evaporation and Crystallization of Concentrate
The use of membrane and ion exchange technologies produces a clean permeate
stream and a reject stream (or concentrate) containing the source water contaminants.
Concentrate generated by membrane water treatment technologies contains sodium and
potassium salts of 36,000 milligrams per liter (mg/L) or more (Tchobanoglous, et al., 2003).
Concentrate generated by ion exchange plants contains sodium (Na+) at average concentrations
between 2,000 and 5,000 mg/L (U.S. EPA, ASCE, and AWWA, 1996). By recovering these
salts, plants can gain a saleable resource and prevent the discharge of the concentrate into surface
waters.
WTPs can use drying beds to recover salts by evaporating the water. Drying beds
are particularly effective in the southern and southwestern parts of the country with moderate to
hot temperatures.
Crystallization of salts from concentrate involves removing enough water to
exceed salt solubility limitations. Once the salt changes phase from dissolved to crystallized
form, it can be readily removed. If the residuals contain mixtures of chemical components, then
additional steps are required to refine the crystallized material prior to sale or reuse.
27
The market price of the coagulant does not include the costs incurred to mitigate any potential environmental
damage that pollutants in coagulants cause the environment when released in the effluent stream of the WTP.
11-9

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
11.2 RESIDUALS TREATMENT
Residuals contain contaminants removed from the source water and treatment
chemicals added by the WTP. Prior to final waste management (e.g., land application, disposal,
or discharge), residuals from the source water treatment operations (e.g., filter backwash water,
coagulation sludge) can be treated on site by the WTP. This subsection is organized by
technologies used by WTPs to achieve the following:
• Separation of solids from water (Section 11.2.1);
• Precipitation of chemicals (Section 11.2.2);
• Increase in oxygen content (Section 11.2.3);
• Removal of chlorine (Section 11.2.4); and
• Adjustment of pH (Section 11.2.5).
Table 11-1 presents the distribution of residuals treatment practices commonly
found at WTPs serving more than 10,000 people. Section 11.2.6 presents nonwater
environmental quality impacts to consider when installing a residuals treatment system.
Table 11-1. Distribution of Residuals Treatment Technologies at Drinking Water
Treatment Plants
Treatment Category
Treatment Unit
Number and Percent of WTPs With the
Treatment Unit in Place
(2,151 WTPs)
Solid/Water Separation
Equalization only
159 (7%)
Clarification
Included with non-mechanical dewatering
Lagoon
Included with non-mechanical dewatering
Thickening
Included with non-mechanical dewatering
Mechanical dewatering
195 (9%)
Non-mechanical dewatering
a
1,413 (66%)
Drying or evaporation
Included with non-mechanical dewatering
Other Residuals Treatment
Chemical precipitation
Not estimated
Aeration to increase oxygen content
Not estimated
Dechlorination
230 (14% of 1,599 plants that disinfect
with free chlorine)
pH Adjustment
Not estimated
No Treatment
No treatment
522 (24%)
Source: Appendix A.
a – Might include equalization.
11-10

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
11.2.1 Solids Removal (Separation of Solids and Water)
The volume and characteristics of the residuals depend on the source water,
drinking water production rate, efficiency of source water treatment, and type of source water
treatment used. Treatment residuals contain naturally occurring suspended and dissolved solids,
as well as precipitated solids generated by chemical treatment. Many WTPs treat residuals to
separate solids from the wastewater.
WTPs can use one or more solids removal processes to treat WTP residuals. For
example, WTPs can separate solids and water using an equalization basin, followed by a gravity
thickener, and finally a centrifuge. At each process in the residual treatment train, additional
separation occurs.
Decreasing the volume of water while increasing solids content is the principle
objective of solids removal systems. The decreased volume reduces landfill requirements and
reduces cost. (Landfills usually charge customers by weight.) “Thickening” and “dewatering” are
solids removal terms that are often used interchangeably. Based on the applicable treatment
techniques, these two practices have many common elements. The discussion that begins with
Table 11-2 describes solid/water separation using the following terminology:
• Thickening: Solids separation by physical means without the significant
application of mechanical devices. Sedimentation (gravity settling) and
dissolved air flotation are examples of drinking water residuals thickening
technologies.
• Mechanical Dewatering: Solids separation by mechanical means.
Pressure filtration and centrifugation are examples of mechanical
dewatering technologies.
• Non-Mechanical Dewatering: Solids concentration by evaporation of the
water. Storage ponds, lagoons, and drying beds are examples of non-
mechanical dewatering.
• Thermal Treatment: Solids concentration by evaporation of the water
using mechanical drying processes.
11-11

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
Table 11-2 presents the range of solids concentrations that typically results from
using various solids removal processes (U.S. EPA, ASCE, and AWWA, 1996).
Table 11-2. Comparison of Solids Removal Technologies: Solids Concentration After
Treatment by Residuals Type
Solids Removal Treatment
Solids Concentration for Treated
Lime Softening Residuals
Solids Concentration for Treated
Coagulation Residuals
Thickening
Gravity Thickening
15–30%
1–3% (low TSS)
5–30% (high TSS)
Flotation Thickening
Not available
2–4%
Gravity Belt
Not available
2.5–4.5%
Mechanical Dewatering
Scroll Centrifuge
55–65%
20–30%
Belt Filter Press
50–60%
1 –20% (Alum)
4–50% (Alum, TSS)
Plate (or Pressure) Filter
55–70%
35–45%
Diaphragm Filter Press
50–70%
30–60% (Alum with lime conditioning)
Non-Mechanical Dewatering
Storage Lagoon
50–60%
7–15%
Sand Drying Bed
50%
20–25%
Source: U.S. EPA, ASCE, and AWWA, 1996.
11.2.1.1 Thickening
The objective of thickening is to increase the solids content of the residuals by
removing a portion of the water. Gravity settling, dissolved air flotation, and gravity belt are the
most common thickening technologies.
Thickening of residuals can take several paths, but the end result is to remove a
portion of the influent water to concentrate the solids for resource recovery. The importance of
having a higher concentration of solids progressing to the next treatment/recovery phase is
reflected in the reduction of the capital and operating costs of the continuing treatment (e.g., an
increase in solids from 3 to 6 percent results in a 50 percent volume reduction which in turn
would reduce capital expenditures associated with the construction of greater wastewater
handling capacity in this residuals treatment phase. An increase in solids concentration can also
11-12

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
facilitate the design and reduce per unit cost associated with wastewater treatment and solids
disposal.
Gravity settling is the term that describes using gravity to separate (thicken) solids
from water. Initially, applications of this technology at WTPs consisted of long, narrow, and
deep tanks with residence times of at least four hours. The industry has since shifted to use of
circular units due to operational difficulties with removing residue from the long, narrow tanks
and advances in engineering design. Figure 11-2 presents a diagram of a circular gravity
thickener. New designs with the same thickening efficiencies have reduced the residence time to
two hours or less (ASCE, 1997). Metal hydroxide (i.e., coagulation) residuals with low TSS
concentrations can be thickened to up to 3 percent solids, while residuals with higher TSS
concentrations can be thickened to as high as 30 percent solids. Lime softening residuals
(carbonate residuals) can be thickened to the range of 15 to 30 percent solids (U.S. EPA, ASCE,
and AWWA, 1996). The number of gravity settling tanks required for residuals treatment
depends on the plant’s treatment volume and the amount of redundancy required. For example, if
the influent solids content is in the 1 to 3 percent range, and a design solids loading rate of 4.0
pounds/day/square foot is used (AWWARF, 1987), a sedimentation tank with a diameter of 30
feet is needed for each million gallons of waste treated.
11-13

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
Source: U.S. EPA, 1987. Design Manual: Dewatering Municipal Wastewater Sludges.
Figure 11-2. Gravity Thickener (U.S. EPA, 2003)
Dissolved air flotation (DAF) is the most common of several flotation separation
technologies. Pressurized air is injected into recycled drinking water and added to the residuals
feed. When the pressure on the injected water is released, it allows the super saturated air to
escape into the residuals as small bubbles that cause turbulence. The small bubbles mix with the
TSS in the residuals stream and adhere to the suspended particles, pushing them to the surface.
The floating material (thickened solids) is then skimmed off. Flotation separation techniques for
drinking water residuals are used more widely in Europe and can generate floating solid residuals
with 3 to 4 percent solids reported (U.S. EPA, ASCE, and AWWA, 1996).
If space is a constraint, or if gravity settling or flotation do not provide the desired
solids thickening, then plants can use a gravity belt thickener as an alternative. Gravity belt
thickeners are constructed from a porous belt (metal mesh) that allows water to drain through the
belt while retaining the solids. The recirculating belt travels through solids removal and wash
sections before returning to service. The design of the belt material and the loading applied
influence separation efficiencies, with solids concentrations for treated metal hydroxides
residuals ranging from 2.5 to 4.5 percent (U.S. EPA, ASCE, and AWWA, 1996). Gravity belts
11-14

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
are simple designs with minimal operator oversight; however, they generate another residuals
stream (wash water), usually require use of a solids conditioner, and require maintenance.
Following the thickening operation, the solids continue to the next solid/water
separation step (i.e., mechanical dewatering). The supernatant from the thickening operation is
recycled or discharged by the plant.
11.2.1.2 Mechanical Dewatering
WTPs commonly follow residuals thickening with mechanical dewatering for
additional volume reduction and concentration of solids. Common mechanical dewatering
technologies used by the WTPs are belt filter presses, plate and frame filter presses, and
centrifuges.
Belt filter presses use pressure to force water out of the residuals through the
porous belt while retaining the separated solids on the belt. Figure 11-3 shows the design of a
belt filter press. Treatment residuals are placed on the dewatering belt and drained in the free
drainage zone. The remaining solids/water are sandwiched between two porous belts and passed
over/under a series of different diameter rollers. The different rollers impart low and high
pressure on the belts, squeezing the additional water from the solids and through the porous belt.
The more extensive the belt travel, the drier the filter cake. Lime softening residuals are good
candidates for this system because their more granular structure can withstand higher pressures.
Using this technology, plants have reported lime filter cake with 50 to 60 percent solids (U.S.
EPA, ASCE, and AWWA, 1996).
Pressure filters (e.g., plate and frame filter press, diaphragm filter press) apply
high pressure to a solid/liquid suspension and force the liquid out while retaining the solids. Plate
and frame filters have a recessed area that receives the pumped influent waste material at
elevated pressures. The filter fabric covering the plates allows the water to escape while retaining
the solids. This is a continuous process until the pressure drop across the filter equals the
pumping pressure and the unit is shut down. The filter is then broken down and manually
cleaned and returned to service. A diaphragm filter press allows WTP operators to vary the
11-15

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
volume in the receiving area. It employs a two-stage filtering process in which the diaphragm is
expanded after initial filtering has been completed. Lime softening residuals have been
dewatered to solids concentrations of 50 to 70 percent using this technique (U.S. EPA, ASCE,
and AWWA, 1996).
Source: U.S. EPA, 1987. Design Manual: Dewatering Municipal Wastewater Sludges.
Figure 11-3. Belt Filter Press (U.S. EPA, 2000a)
Centrifugal separators use centrifugal force to separate suspended solids from
water. The amount of force applied to the waste stream solids depends on the centrifuge’s
rotational speed. The force applied and the centrifuging time determine separation effectiveness.
As the industrial application of centrifuges increases in size, so do the operational problems and
energy costs. The solid bowl centrifuge is the principal type of centrifugal separator used to
dewater treatment residuals. The bowl centrifuge has two moving parts: the bowl and the scroll.
As centrifugal force pushes the solids to the edge of the spinning bowl, a rotating scroll moves
the dewatered solids along a horizontal axis to a collection point. Centrifuges perform better with
the addition of a conditioning agent, thus they are rarely operated without the addition of a
polymer to the residual suspension (U.S. EPA, ASCE, and AWWA, 1996).
11-16

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
Table 11-3 lists example cake solids concentrations that have been achieved by
mechanical dewatering operations performed in a laboratory (U.S. EPA, ASCE, and AWWA,
1996).
Table 11-3. Laboratory Results for Mechanical Dewatering Operations for Various
Drinking Water Treatment Residuals
Residuals
Specific Gravity
of Particles
Solids Concentration
After Gravity
Thickening
Solids Concentration After
Mechanical Dewatering
Centrifuge
Pressure Filter
Lime Softening Sludge (low
Magnesium [Mg])
1.19
28.5%
60.6%
69.5%
Iron Sludge
1.16
26.0%
55.6%
64.6%
Ferric Hydroxide
1.07
7.2%
28.2%
36.2%
Lime Sludge (high Mg)
1.05
5.6%
24.8%
34.6%
Aluminum Hydroxide
1.03
3.6%
19.0%
23.2%
Source: U.S. EPA, ASCE and AWWA, 1996.
11.2.1.3 Non-Mechanical Dewatering
Two types of non-mechanical dewatering are discussed in this section: storage
lagoons and drying bed operations. Both are often used at the end of the residuals treatment train.
Storage Ponds and Lagoons
WTPs can collect and hold treatment residuals in settling ponds, tanks, or lagoons
to separate solids. Plants can allow solids settling prior to further solids separation (e.g.,
thickening or mechanical dewatering) or discharge. In addition, lagoons and ponds can serve as
long-term waste disposal. Since the separation occurs without physical means, the use of
lagoons, ponds, and settling tanks is considered a non-mechanical dewatering process.
WTPs collect residuals in storage ponds and lagoons and allow long-term
sedimentation and compaction to separate the solids from the water. For metal hydroxide
residuals like aluminum and iron (from coagulation) that are retained in a pond or lagoon for a
month, solids concentrations of 10 percent in the settled sludge are common. For lime softening
sludges, solids concentrations of 20 percent in the settled sludge are common (ASCE, 1997).
11-17

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
Storage ponds and lagoons are long-term residuals treatment approaches that
require periodic draining, cleaning, and maintenance. In addition, application of this residuals
treatment method depends on the land available, evaporation rates (if no further discharge or
recycling), and any ground water contamination concerns. This use of storage ponds might result
in no discharge from the WTP; however, some WTPs perform intermittent discharges of
overflows from the tanks.
Drying Beds
Drying bed technologies share a common design concept: the cover material (bed)
is installed over an under-drain consisting of gravel and perforated pipe. Drying bed technologies
differ in the type of supporting material used for the bed surface (e.g., sand) and in whether
external forces such as vacuum are used to promote the separation of the solids (see Figure 11-
4). Initially, water percolates through the bed and is collected by the under-drain and discharged.
Additional dewatering then occurs via evaporation. The rate of evaporation depends on the local
climate, the solids characterization in the residuals, and the extent of external drainage
enhancement. Thin layers dry faster than thick layers, but result in higher operating costs.
The following non-thermal drying bed technologies are used to reduce the
moisture content in WTP residual solids:
• Sand drying beds dewater residuals by gravity drainage, followed by
evaporation. Water drains through the sand and exits through the under-
drain.
• Freeze-assisted sand beds are sand drying beds where the residuals are
applied and then allowed to freeze (either naturally or mechanically). By
freezing and then thawing the residuals, the solids become compressed
together, more granular, and easier to dewater. WTPs use this technique
for alum residuals, which have a gelatinous consistency that makes them
difficult to dewater without the added freezing step.
• Vacuum-assisted systems apply negative pressure to promote the
percolation of the free water through the bed, thus speeding the drying
process.
11-18

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
• Solar drying beds can be used in specific geographic locations where the
climate is sufficiently hot and dry (e.g., southwestern United States) to
quickly dry the residuals. “Greenhouse” solar drying beds can also be used
in less sunny areas, but they are not currently widespread.
Lime solids concentrations as high as 50 percent have been achieved using drying
beds. Alum residuals might require the addition of a chemical conditioner prior to drying. The
solids content after the drying bed has been reported as high as 25 percent (U.S. EPA, ASCE,
and AWWA, 1996).
Sand
Gravel
Splash
Slab
Side
Wall
Gate
Collection
System
Drainage
Sludge
Figure 11-4. Sand Drying Bed Section (U.S. EPA, ASCE, and AWWA, 1996)
11.2.1.4 Thermal Drying
The final step in a residuals treatment train might be thermal drying. Thermal
drying is not widely used by the industry because the costs of the technology are more than the
costs savings that result from reduced residuals volume. In general, WTPs employ this
technology to solve problems with pathogen control, odor control, and storage problems rather
than to achieve solids/water separation alone. Thermal drying operations include direct fired
systems (rotary kiln, fluidized bed, low temperature desorption), indirect fired (heated coils), and
infrared radiation.
11-19

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
11.2.2 Chemical Precipitation
Chemical precipitation removes dissolved metals from wastewater by the addition
of a precipitating reagent. The reagent reacts with the metal ions and creates insoluble forms of
the metal. This type of residuals treatment is applicable to aqueous waste streams, such as filter
ion exchange backwash and rinse and membrane desalination concentrates. The most common
precipitating reagent is hydroxide; WTPs can add lime, quicklime, soda ash, or caustic soda to
the residuals to introduce the hydroxide ions. Depending on the metals present in the residuals,
sulfide and ferrous salt can also be used. Chemical precipitation of the residuals can be used to
remove aluminum, antimony, arsenic, cadmium, chromium, copper, iron, lead, mercury,
selenium, silver, thallium, or zinc. (U.S. EPA, 1993)
Equipment needed to perform chemical precipitation includes a stirred vessel
reactor and clarifier. WTPs can add coagulants to aid solid settling. This treatment process
results in: 1) a clean supernatant stream that is recycled or discharged, and 2) clarifier sludge.
The clarifier sludge can be dewatered prior to disposal. (U.S. EPA, 1993)
11.2.3 Increased Oxygen Content by Aeration
Drinking water plants use aeration to treat both the source water and residuals
streams. Aeration increases the oxygen content in the water. The dissolved oxygen concentration
in the water indicates the amount of oxygen used by biological components and provides a
qualitative measure to judge the relative purity of the residuals stream. To control biological
oxygen demand discharges and increase dissolved oxygen levels, WTPs add oxygen to residuals
prior to discharge.
11.2.4 Dechlorination
Residual chlorine in WTP discharges is toxic to many kinds of aquatic life and
can react with organic materials in the receiving water to form carcinogenic trihalomethanes and
organochlorines, including chloramines. Chloramines are highly toxic to fish and other
organisms that live in water. Dechlorination removes the free or total combined chlorine residual
11-20

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
remaining after disinfection through the addition of sulfur chemicals such as sulfur dioxide,
sodium sulfite, sodium bisulfite, sodium metabisulfite, and sodium thiosulfite. Carbon adsorption
can also be used for total dechlorination; however, this process is typically more expensive.
Dechlorination requires an adequate control system to reduce residual chlorine to
near-zero levels of residual chlorine without overdosing with sulfite. Too much sulfite can result
in sulfate formation, which suppresses oxygen content and lowers the pH of the treatment
residuals (U.S. EPA, 2000b).
As presented in Section 3.3.2.2, EPA’s survey found that 93 percent of WTPs in
the target population perform primary disinfection (i.e., 2,002 of the 2,151 WTPs). Most WTPs
(1,917 of 2,002) use free chlorine or chloramines for primary disinfection (1,599 and 318 plants
respectively). EPA’s survey found that only 230 WTPs perform dechlorination.
Costs for a dechlorination system depend on the particular conditions at the WTP.
Cost considerations include capital costs (equipment, installation, and labor), operation and
maintenance costs, and type of dechlorination chemical (U.S. EPA, 2000b).
11.2.5 pH Adjustment
As a result of treatment chemical addition, the source water pH is altered during
treatment operations to improve treatment performance. Ecosystems are more vulnerable than
humans to changes in pH—for example, small changes can affect reproductive patterns and
longevity. NPDES permits typically require the pH of residuals discharges to range between 6
and 9. To adjust the pH, WTPs add acids to lower the pH and bases to raise the pH. Chemicals
used by WTPs meet certified purity standards.
11.2.6 Nonwater Quality Environmental Impact Considerations
Eliminating or reducing one form of pollution may create or aggravate other
environmental problems. When reviewing whether to install a residuals treatment system, WTPs
11-21

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
and permit writers must look at the nonwater quality environmental impacts to determine any
adverse effects on the environment.
11.2.6.1 Air Pollution and Control
The majority of impurities removed during source water treatment include
suspended solids, metals, synthetic organic chemicals, and microbes. EPA does not expect
suspended solids, metals, or microbes to escape and become air pollutants during residuals
treatment. Air stripping of volatile organics is a residuals treatment option available to WTPs;
however, the use of air stripping is infrequent. Materials handling operations may generate
fugitive emissions, and these emissions can be managed by installing a proper ventilation system
or dust suppression system.
Any increased air emissions as a result of installing residuals treatment would be
primarily from the electric power generation facilities providing any additional energy and
increased truck traffic due to additional sludge hauling.
11.2.6.2 Solid Waste Generation and Disposal
WTPs that treat large volumes of source water can generate large volumes of
residuals. Plants have several options for handling the sludge/slurries produced by source water
treatment. Options range from recycle/reuse to direct discharge. Recycling, discharging to a
landfill, and performing land application are the most common approaches (U.S. EPA, 2008).
Residuals from WTPs are typically not hazardous and can be accepted by landfills
or managed via land application. Treatment and disposal methods for residuals may vary among
WTPs and are based on the characteristics of the waste. The volume and characteristics of the
residuals generated by WTPs are discussed in Section 7 of this document.
11.2.6.3 Energy Requirements
The operation of the residuals treatment technology and operation of pumps to
recycle residual streams would require additional energy. Total energy requirements for residuals
11-22

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
treatment technologies are not expected to create a large impact. Incremental energy costs may
be incurred by the installation of residuals treatment technology or other practices.
11.3 DISPOSAL PRACTICES FOR TREATMENT RESIDUALS
This subsection summarizes the typical disposal practices for residuals, including
land application (Section 11.3.1) and land disposal via landfilling or deep well injection (Section
11.3.2). These disposal practices help reduce or eliminate discharges to surface water and
POTWs. As such, they can be employed to help WTPs become zero discharging facilities.
11.3.1 Land Application of Residuals
After separating the solids from the wastewater and recovering usable materials,
WTPs typically manage residual solids by land application or disposal in landfills (see Section
11.3.2). Land application involves spreading residuals on the land and cultivating it into the soil.
The application of residuals onto land depends on the crop being grown,
chemistry of the soil, and sludge properties. Land application typically occurs with lime
softening sludge, and to some extent coagulation sludge (e.g., alum sludge). Lime softening
sludge can be used on farm land in place of commercial products to neutralize soil pH. Alum
sludge does not benefit the soil and is used only for filler material. The ideal land application of
WTP residuals occurs on non-food chain crops, mine reclamation areas, and forests (U.S. EPA,
1993).
Disadvantages of land application might exist depending on the properties of the
residuals. For example, land application can result in increased concentration of metals in the soil
(and possibly ground water). Application of aluminum and iron hydroxide sludge from
coagulation can result in the adsorption of phosphorus from the soil to the applied residuals,
resulting in less productive soil (U.S. EPA, 1993).
Land application requires large tracts of land and additional supporting
infrastructure (tractor, pipes, lagoon, etc.). Further, ground water protection must also be
11-23

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
addressed. If 1,000 (dry) pounds of residuals are produced daily, about 300 acres and about
$50,000 in annual operating expenses are required (U.S. EPA, ASCE, and AWWA, 1996).
WTPs can transport the residuals for offsite land application via tanker or truck.
Residuals managed by land application typically contain less than 15 percent
solids. There must be sufficient liquid in the residuals to form pumpable slurry. Land application
methods include spraying from trucks or a sprinkler system, injecting into the subsurface, or
discharging the slurry onto a selected field. Dewatered residual sludge can be spread on the land
(U.S. EPA, 1993).
Land application of membrane desalination concentrates is not as common as
application of residual sludge. However, if desalination concentrates are applied to land, WTPs
use percolation ponds, rapid infiltration basins, or landscape/crop irrigation (Malmrose, et al.,
2004).
11.3.2 Disposal of Residuals to Landfills or Deep Injection Wells
Landfills for residuals can be either monofills (which contain one kind of waste)
or municipal sanitary landfills (which contain many different kinds of waste). Disposal fees are
usually based on weight of material presented for disposal and vary with different locations
around the country. EPA regulates landfill disposal under the Resource Conservation and
Recovery Act (RCRA).
In addition to landfills, WTPs can dispose of residuals using subsurface, or deep
well, injection. Concentrates from membrane desalination can be disposed of through this
practice, which is commonly performed by plants in Florida (Malmrose, et al., 2004). EPA
regulates deep well injection disposal under its Underground Injection Control (UIC) program.
11.4 WASTEWATER DISCHARGES OF TREATMENT RESIDUALS
Wastewater from WTPs, such as filter backwash water, can be recycled to the
head of the source water treatment plant or evaporated from residual solids. Solids (or slurries)
11-24

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
from WTPs, such as lime softening sludge and coagulation sludge, can be dewatered and
disposed of in a landfill or managed by land application. In some cases, WTPs opt to discharge
treatment residuals either directly to waters of the United States or indirectly through publicly
owned treatment works (POTWs).
Direct discharge to surface waters is the most common waste management
method for conventional filtration and precipitative softening plants. Some of these WTPs are
also able to achieve zero discharge using recycling, land application, and landfill disposal.
Indirect discharge is common for WTPs co-located with POTWs (i.e., operated by
a local municipality) (U.S. EPA, 1993). Most membrane desalination plants are indirect
dischargers or zero dischargers.
Some of the best discharge practices that might be included in NPDES permits or
implemented by WTPs include the following:
• Limiting discharge flow rate. Rather than allowing batch discharges,
NPDES permits can require WTPs to slowly discharge residuals into the
receiving stream. Slowly discharging the residuals allows dilution in the
receiving stream and minimizes the impacts of the pollutant discharge.
• Prohibiting discharges of solid residuals unless land-based use/disposal
options are not feasible and/or WTPs demonstrate discharge does not
degrade receiving water quality.
• Requiring that solids disposal from periodic cleaning of settling basins be
land-based to avoid large batch discharges to the receiving stream.
• Prohibiting discharges of chlorinated backwash (or other waste streams)
unless the WTP demonstrates that the receiving water-quality standards
can be met at all times.
• Equalizing
28
the residuals discharge to avoid large batch discharges of
pollutants. The WTP collects residuals in a tank, basin, or other device and
discharges at a controlled flow rate over time. This practice can be used
for filter backwash water (generated at very high flow rates for short
28
Equalization is the practice of collecting residuals in a tank, basin, or other device for later treatment or discharge
at a controlled flow rate.
11-25

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
periods of time) and ion exchange regeneration waste streams (also
generated at intermittent times) (AWWARF, 1987).
11.5 REFERENCES
American Society of Civil Engineers (ASCE)/American Water Works Association (AWWA),
1997. Water Treatment Plant Design, 3
rd
Edition, New York: McGraw-Hill. Document Control
Number (DCN) DW00961.
American Water Works Association Research Foundation (AWWARF), 1987. Water Treatment
Plant Waste Management. Prepared by Environmental Engineering and Technology, Newport
News, VA, for AWWARF, Denver, CO. DCN DW00186.
Malmrose, et al., 2004. Paul Malmrose, Jim Lozier, Michael Mickley, Robert Reiss, Jerry
Russell, James Schaefer, Sandeep Sethi, Jennifer Manuszak, Robert Bergman, and Khalil Z.
Atasi, Residual Management Research Committee Subcommittee on Membrane Residual
Management, “2004 Committee Report: Residuals Management for Desalting Membranes,”
Jour. AWWA, 96:12:73. American Water Works Association (AWWA), December 2004. DCN
DW00032.
Tchobanoglous, et al., 2003. George Tchobanoglous, Franklin L. Burton, H. David Stensel,
Wastewater Engineering Treatment & Reuse, 4
th
edition. Metcalf & Eddy, Inc., New York:
McGraw-Hill. DCN DW00871.
U.S. Environmental Protection Agency (EPA), 1992. Facility Pollution Prevention Guide (EPA-
600-R-92-088). Office of Solid Waste, Washington, DC. DCN DW00885.
U.S. EPA, 1993. Large Water System Byproducts Treatment and Disposal Cost Document (EPA
811-D-93-002). Office of Water, Washington, DC. DCN DW00058.
U.S. EPA, ASCE, and AWWA, 1996. Technology Transfer Handbook: Management of Water
Treatment Plant Residuals (EPA 625-R-95-008). Office of Research and Development,
Washington, DC. DCN DW03736.
U.S. EPA, 2000a. Biosolids Technology Fact Sheet: Belt Filter Press (EPA 832-F-00-057).
Office of Water, Washington, DC. [Internal Reference: U.S. EPA, 1987. Design Manual:
Dewatering Municipal Wastewater Sludges.] DCN DW00900.
U.S. EPA, 2000b. Wastewater Technology Fact Sheet: Dechlorination (EPA 832-F-00-022).
Office of Water, Washington, DC. DCN DW00678.
U.S. EPA, 2002a. Filter Backwash Recycling Rule: Technical Guidance Manual (EPA 816-R-
02-014). Office of Ground Water and Drinking Water, Washington, DC. DCN DW00064.
U.S. EPA, 2003. Biosolids Technology Fact Sheet: Gravity Thickener (EPA 832-F-03-022).
Office of Water, Washington, DC. [Internal Reference: U.S. EPA, 1987. Design Manual:
Dewatering Municipal Wastewater Sludges.] DCN DW00901.
11-26

Drinking Water Industry Report Section 11 – Technologies & Practices for Residuals
U.S. EPA, 2005. Drinking Water Treatment Facility Site Visit Report: James J. Corbalis Water
Treatment Plant. Office of Water, Washington, DC. DCN DW00178.
U.S. EPA, 2009. Drinking Water Survey Response Database – Technical Data for the 2006
Drinking Water Industry Questionnaire. Office of Water, Washington, DC. DCN DW03790.
Yohe, Thomas L. et al., 2006. White Paper: The Effect of Low Uniformity Coefficient Anthracite
on Dual Media Filtration. The F.B. Leopold Company, Inc., Bryn Mawr, PA, March 20, 2006.
DCN DW03779.
11-27

SECTION 12
TREATMENT TECHNOLOGY COST CONSIDERATIONS FOR
RESIDUALS THICKENING AND DEWATERING
As part of the drinking water industry review, EPA investigated technologies
available to reduce residual discharges from the most common types of water treatment plants
(WTPs) that discharge to surface waters. EPA evaluated the factors that affect the cost of
installing and operating residuals treatment systems from conventional filtration (i.e.,
coagulation and filtration) and precipitative softening plants since these plants are the most
prevalent across the country. EPA did not analyze options for treating residuals from other types
of plants (e.g., ion exchange, adsorption, or membrane desalination). This section summarizes
EPA’s findings on costs and provides references to assist permit writers in estimating the costs
for technology options. An example of costing analysis performed for a conventional filtration
WTP is contained in the report Technical Analysis for Determination of Technology-Based
Permit Limits for the Guaynabo Drinking Water Treatment Facility NPDES No. PR0022438
(U.S EPA, 2009).
Section 12.1 presents a typical residuals treatment system which EPA used as part
of its costing review. Section 12.2 provides background on cost data sources including cost
models reviewed by industry experts and recent data provided by an industry trade association.
Sections 12.3 and 12.4 summarize the determination of system size requirements and estimation
of approximate costs for specific elements of the residuals treatment system.
12.1 RESIDUALS THICKENING AND DEWATERING TREATMENT TRAIN
Residuals from softening and conventional filtration plants include sedimentation
basin underflow and spent filter backwash (see Figure 6-1). These residuals may be treated by
various dewatering processes. As described in Section 11.2, WTPs can use one or more solids
removal processes to dewater WTP residuals. At each process in the residual treatment train,
additional separation occurs.
12-1

Drinking Water Industry Report Section 12 – Treatment Technology Cost Considerations
This report identifies cost considerations for the typical residuals treatment train
illustrated in Figure 12-1. The residuals treatment train includes the following processes: spent
filter backwash (SFBW) equalization basins and clarifiers, thickeners that further dewater
clarifier underflow and treat sedimentation basin underflow from the source water treatment
plant, centrifuges to further dewater underflow from the thickener, and final sludge handling
prior to disposal. The figure does not show the source water treatment operations, only the
treatment of residuals.
WTPs produce finished drinking water and generate residuals during source water
treatment. Residuals from lime softening, coagulation, and filtration processes include filter
backwash and sedimentation basin sludge. In the typical residuals treatment train, SFBW is
pumped to equalization basins followed by a clarifier. Clarifier overflow can be discharged or
recycled, as shown by the dashed line. Clarifier underflow is pumped to a thickener.
The thickener receives the SFBW clarifier underflow, sludge from the WTP’s
sedimentation basin, and the water that is removed during the dewatering step. Thickener
overflow can be either recycled or discharged, as shown by the dashed line. Thickener underflow
is pumped to dewatering.
In Figure 12-1, dewatering is accomplished using centrifuges. Centrate, the water
that is removed from sludge in the centrifuge is shown returning to the thickener. Dewatered
solids are stored and ultimately disposed.
12-2

Drinking Water Industry Report Section 12 – Treatment Technology Cost Considerations
Figure 12-1. Residuals Treatment Technology Train
Spent Filter
Backwash
Equalization
Basins
(
2
)
Spent Filter
Backwash
Holding
Tank
Overflow
Recycle or Discharge
Polymer
Underflow from WTP
Sedimentation Basins
Overflow
Polymer
Off
-
Site Disposal
Centrate Recycled from Dewatering
Spent Filter
Backwash
Clarifier
Thickener
Polymer
Biosolids
Storage
Bins
Lime
Dewatering
Centrifuges
(
2
)
Dewatered
Sludge Cake
Source
Water
Treatment
Plant
Source
Water
Treatment
Plant
12-3

Drinking Water Industry Report Section 12 – Treatment Technology Cost Considerations
12.2 COST DATA SOURCES IDENTIFIED
EPA identified several primary sources of data to assess the cost of installing and
operating residuals treatment systems. First, EPA sought the opinion of industry stakeholders and
experts to review and characterize cost information (see Section 2.6). Second, EPA used data
provided by the American Water Works Association (AWWA, 2008), an industry trade
association. Third, EPA incorporated information on its forthcoming Work Breakdown Structure
(WBS) cost models for drinking water treatment technologies.
12.2.1 Drinking Water Treatment Technology Review Group
EPA sought the opinion of a broad range of stakeholders to review documents
that summarize the major technical and engineering issues related to the management of drinking
water treatment residuals. Goals for this review included the following:
• Characterization of typical residuals;
• Identification of pollutants of concern;
• Identification of pollution prevention and treatment technologies for
residuals;
• Evaluation of 1993 and 1987 cost estimates developed by EPA and
AWWA, respectively, for these residuals treatment technologies; and
• Application of prevention and treatment technologies.
From 2005 through 2007, EPA held several meetings and provided stakeholders
with various technical papers to review. EPA developed the document entitled, Identification of
Technology Options (U.S. EPA, 2006), which included possible technology options to control
residuals discharges and cost considerations for these options. EPA received comments on the
technology options document and developed an input summary document (U.S. EPA, 2007).
The Identification of Technology Options document (U.S. EPA, 2006) included a
comparison of costing data sources developed for WTP residuals. EPA reviewed the following
costing data sources:
12-4

Drinking Water Industry Report Section 12 – Treatment Technology Cost Considerations
• EPA, 1993: EPA developed and presented drinking water residuals
treatment costs in the Large Water System Byproduct Treatment and
Disposal Cost Document (U.S. EPA, 1993). EPA also presented these
costs in Chapter 11 of the Office of Research and Development (ORD)
Technology Transfer Handbook: Management of Water Treatment
Residuals (U.S. EPA, ASCE, AWWA, 1996). This source is referenced as
EPA's 1993 costs.
• ERG, 2006: ERG, 2006 included a summary of information from early
drafts of EPA's WBS cost models. This source has been superseded by up-
to-date information provided directly by EPA (see Section 12.2.3).
• AWWA 1987: AWWA developed and presented residuals treatment costs
in the handbook Water Treatment Plant Waste Management (AWWA,
1987).
Both EPA and AWWA estimated costs for several treatment technologies for
residuals. Table 12-1 references specific sections for the residuals management cost equations
that are available from EPA’s 1993 document and AWWA’s 1987 document. AWWA and EPA
have also estimated lagoon costs and costs for evaporation ponds/sand drying beds. However,
their use as a treatment option is highly dependent on weather/climate and the availability of land
and their cost curves are highly dependent on land costs. Therefore, those options were not
included in the costing review summarized in the Identification of Technology Options
document.
12-5

Drinking Water Industry Report Section 12 – Treatment Technology Cost Considerations
Table 12-1. Available Residuals Management Cost Equations
Management Options
1993 EPA Section
1987 AWWA Section
Gravity Thickening
4.4
4.3.3
Chemical Precipitation
5.5
Not Included
Sludge Conditioning
Polymer feed system and feed included with
filter press costs.
4.4.2
Sludge Pumping
Thickened sludge pumping costs to the filter
press are included in the filter press costs.
Assumes waste streams flow by gravity
from the treatment plant to the settling tank.
4.5.2
Mechanical Dewatering – Pressure Filter
Press
6.5
4.7.3
Mechanical Dewatering –Centrifuge
(Scroll / Decanter)
6.10
4.6.3
Mechanical Dewatering – Belt Filter
Press
Not included
4.9.3
Non-Mechanical Dewatering – Lagoon
(lime softening sludge)
7.5
4.11.3
Non- Mechanical Dewatering – Lagoon
(alum sludge)
7.6
4.11.3
Evaporation Ponds / Sand Drying Beds
8.5
4.10.3
POTW Discharge
9.5
Not included
Direct Discharge
10.5
Not included
Land Application – liquid sludge
11.5
Not included
Land Application – dewatered sludge
11.7
Not included
Non-hazardous Waste Landfill – off site
12.4
Not included
Non-hazardous Waste Landfill – on site
12.6
Not included
Hazardous Waste Landfill
13.4
Not included
Radioactive Waste Disposal
Not included
Not included
Deep Well Injection
Not included
Not included
Chemical Recovery
Not included
4.12
Source: U.S. EPA, 2006.
POTW—Publicly Owned Treatment Works.
12.2.2 AWWA 2008 Cost Estimates
AWWA sponsored a report entitled Costing Analysis to Support National
Drinking Water Treatment Plant Residuals Management Regulatory Options (AWWA, 2008).
AWWA estimated costs to install and operate a typical residuals treatment system at model
plants and reviewed its estimates compared with actual installations. The resulting report
presents a series of cost curves showing cost relative to population served, WTP type
(conventional filtration or lime softening), and solids loading. By developing several of these
12-6

Drinking Water Industry Report Section 12 – Treatment Technology Cost Considerations
cost estimates for a range of plant sizes, this study was able to capture the range of costs
associated with implementing residuals management at WTPs plants across the country.
12.2.3 EPA’s Work Breakdown Structure (WBS) Cost Models
EPA has developed its draft WBS cost estimating models for drinking water
treatment technologies andanticipates public release of selected models in 2012.
29
The WBS
models are spreadsheet-based engineering models for individual treatment technologies, linked
to a central database of component unit costs. Under the WBS approach, a treatment technology
is broken down into discrete components that can be measured for the purpose of estimating
costs. The components include capital equipment (e.g., tanks, vessels, pipes, and instruments)
and operational expenditures (e.g., annual expenditures on labor, chemicals, and energy).
By adopting a WBS-based approach to identify the components that should be
included in a cost analysis, the models produce a transparent and comprehensive assessment of
the capital and operating costs for a treatment system.
Instead of presenting a series of total cost curves, the WBS models estimate the
cost of an individual treatment plant, including residuals management, at the level of line-item
detail for individual pieces of equipment (e.g., clarifiers, piping, valves, instrumentation and
system controls). Although the models estimate total cost for the entire treatment process, critical
components of residuals management can easily be identified in the line-item output list. There
are separate models for several conventional and emerging water treatment technologies.. The
residuals management options available in each model are specific to the technology being
modeled, driven by the types of residuals generated, their quantity, the frequency of generation
(e.g., intermittent versus continuous), and their characteristics.
EPA subjected the individual models to a process of external peer review by
nationally recognized technology experts. EPA also has conducted benchmarking, comparing the
model results to actual capital and O&M costs for existing drinking water treatment systems.
29
For updates on the status of the WBS models, please check the EPA webpage at:
http://water.epa.gov/scitech/wastetech/guide/treatment/index.cfm.
12-7

Drinking Water Industry Report Section 12 – Treatment Technology Cost Considerations
12.3 TREATMENT UNITS: DESCRIPTION AND CAPACITY
Many variables affect the cost of installing and operating a residuals treatment
system, but the variable driving cost is capacity. The capacity requirements for treatment units
determine how large they are and how many are required, which is by far the determining factor
in the cost of residuals treatment. The factors that affect the capacity requirements are solids
content and residuals flow rate (AWWA, 2008; U.S. EPA, ASCE, AWWA, 1996).
This section discusses how to estimate capacity requirements for the residuals
treatment system shown in Figure 12-1, which includes the following treatment units:
• SFBW equalization tanks;
• SFBW clarifier(s);
• Gravity thickener(s);
• Dewatering holding tank(s);
• Dewatering centrifuges; and
• Ancillary equipment, including pumps, associated piping, control devices,
and buildings to house equipment as necessary.
By estimating the flow of residuals from source water sedimentation, source water
filtration, and residuals dewatering, the capacity requirements of a residuals treatment system can
be determined and costs can then be estimated.
12.3.1 Typical Ranges of Solids Content and Flow in Residuals from Conventional
Filtration and Softening Plants
As much as the capacity requirements of treatment units drive costs, it is the
solids content and flow of residuals that drive the capacity requirements (AWWA, 2008; U.S.
EPA, ASCE, AWWA, 1996). For example, if the source water for a treatment system is lake
water, the residuals flow and solids content may be low. If the source water for a treatment
system is from a river that receives large sediment loads, the residuals flow and solids content
12-8

Drinking Water Industry Report Section 12 – Treatment Technology Cost Considerations
will be higher. The residuals treatment system would be smaller in capacity and less expensive to
build and operate for the lake water WTP than for the river WTP, assuming their finished water
productions are similar.
In its 2008 study, AWWA estimated a range of residuals production, based on
population served. The study found that plant flow rate (finished drinking water) averages 150
gallons per capita per day (gpcpd) (AWWA, 2008). This corresponds well with results from
EPA’s data collection, as shown in Table 3-4 (on an annual basis). AWWA compiled ranges of
residuals production for the various plant sizes using unit residuals production data from EPA’s
Information Collection Rule (ICR) database, shown in Table 12-2. These ranges can be used to
help estimate capacity requirements for treatment units.
Table 12-2. Ranges of Residuals Production Estimated for AWWA 2008 Study
Population
Average Flow Rate
for WTP
(MGD)
Unit Residuals Production
Design Daily Residuals Production
(dry)
Low
a
(lb/mg)
High
a
(lb/mg)
Low (lb/day)
High (lb/day)
Coagulation and Filtration
13,000
2.0
120
539
351
1,577
30,000
4.5
810
3,638
70,000
10.5
1,890
8,489
110,000
16.5
2,970
13,340
175,000
26.3
4,725
21,223
265,000
39.8
7,155
32,138
400,000
60.0
10,800
48,510
650,000
97.5
17,550
78,829
1,000,000
150.0
27,000
121,275
12-9

Drinking Water Industry Report Section 12 – Treatment Technology Cost Considerations
Table 12-2. Ranges of Residuals Production Estimated for AWWA 2008 Study
Population
Average Flow Rate
for WTP
(MGD)
Unit Residuals Production
Design Daily Residuals Production
(dry)
Low
a
(lb/mg)
High
a
(lb/mg)
Low (lb/day)
High (lb/day)
Precipitative Softening
13,000
2.0
1,278
3,151
3,738
9,217
30,000
4.5
8,627
21,269
70,000
10.5
20,129
49,628
110,000
16.5
31,631
77,987
175,000
26.3
50,321
124,071
265,000
39.8
76,201
187,878
400,000
60.0
115,020
283,590
650,000
97.5
186,908
460,834
1,000,000
150.0
287,550
708,975
Source: AWWA, 2008.
MGD—million gallons per day.
lb - pound.
mg - milligram.
a - The AWWA used the median solids concentration from lake sources in the ICR database for the “low” unit
residuals production and the 90th percentile solids concentrations from river sources in the ICR database for the
“high” residuals production.
12.3.2 Spent Filter Backwash Equalization and Clarifier Capacity
As described in Section 7, WTPs typically backwash filters to clean them,
generating SFBW, including filter-to-waste,
30
typically at a rate of 2 to 5 percent of the total
plant production volume (U.S. EPA, ASCE, AWWA, 1996). For example, a WTP producing 1
MGD of finished water would generate approximately 20,000 to 50,000 gallons per day (GPD)
of SFBW. Typically, WTPs backwash one filter at a time, which results in spikes of SFBW sent
to residuals treatment. SFBW equalization tanks provide a consistent, lower flow through the
residuals treatment system. The lower flow lowers the required treatment capacity, and the cost
to install and operate the overall treatment system decreases (AWWA, 2008).
In their 2008 study, the AWWA found that WTPs could estimate the optimal
capacity required for SFBW equalization based on a SFBW recycle flow rate of 6 percent of the
30
Filter-to-waste is the initial permeate production when a filter is brought back online following backwashing, and
is part of the backwash waste stream.
12-10

Drinking Water Industry Report Section 12 – Treatment Technology Cost Considerations
total WTP flow rate (AWWA, 2008).
31
Also, the AWWA found that WTPs should install at least
two equalization basins to provide redundancy. For a smaller plant, a single tank may meet
capacity requirements; however, the plant would require an additional tank to provide backup.
Table 12-3 summarizes optimal capacities for SFBW equalization tanks from AWWA’s 2008
study. The table presents both average and peak flow rates for the WTP, which are used when
sizing equipment and determining required volume capacity.
Table 12-3. SFBW Equalization Basin Capacity
Population
Served
Plant Flow Rate
Optimal Capacity
b
Number of
Basins
Basin
Diameter
a
Average (mgd)
Peak (mgd)
MGal
ft
3
13,000
2.0
3.9
0.22
28,845
2
38
30,000
4.5
9.0
0.44
58,151
2
53
70,000
10.5
21.0
0.97
129,336
2
79
110,000
16.5
33.0
1.45
194,365
2
97
175,000
26.3
52.5
2.03
272,027
2
94
265,000
39.8
79.5
2.44
326,830
2
102
400,000
60.0
120.0
2.88
385,001
2
111
650,000
97.5
195.0
2.16
288,390
2
96
1,000,000
150.0
300.0
1.43
190,896
2
96
Source: AWWA, 2008.
a - Basin height was limited to less than 20 feet, and basin diameter was limited to 150 feet. AWWA’s analysis was
based on actual residuals treatment plant installations and standard assumptions used for engineering design.
b - Design capacity based on 6 percent of plant flow rate (peak capacity is used to set maximum size needed). As
plant flow increases, required capacity decreases. That is, the backwash:plant flow ratio decreases as plant flow
increases. AWWA’s analysis was based on actual residuals treatment plant installations and standard assumptions
used for engineering design.
As shown in Figure 12-1, water from the SFBW equalization basins can be treated
through clarifiers to initially remove solids. The optimal capacity of SFBW clarifiers can be
derived from the same flow rates shown in Table 12-3. The cost data sources discussed in this
section provide further details on clarifier sizing, polymer feed rates, and design assumptions.
After equalization and clarification, the treated SFBW overflow is either recycled or discharged.
SFBW clarifier underflow typically contains 1 to 3 percent solids, and further dewatering is
necessary, hence the thickener (U.S. EPA, ASCE, AWWA, 1996; Bosgraaf, 2005).
31
The general assumption of 6 percent is based on review of plant data that show recycle to be approximately 6
percent of spent filter backwash water and recommendations by The Partnership for Safe Water and EPA’s Filter
Backwash Recycling Rule (recycle no more than 5 to 10 percent of total plant flow).
12-11

Drinking Water Industry Report Section 12 – Treatment Technology Cost Considerations
12.3.3 Gravity Thickener Capacity
As shown in Figure 12-1, the typical gravity thickener receives clarified SFBW
and sedimentation tank underflow from the WTP. Gravity thickeners increase the solids content
of sludge and further remove solids from the residuals wastewater stream. The thickened sludge
is pumped to the dewatering portion of the residuals treatment train, and the thickener overflow
is either recycled or discharged.
In thickened lime sludge, the solids content ranges from 15 to 30 percent (U.S.
EPA, ASCE, AWWA, 1996; AWWA, 2008); thickened coagulant sludge tends to have a solids
content between 2 to 10 percent (U.S. EPA, ASCE, AWWA, 1996; AWWA, 2008).
Gravity thickener capacity requirements depend on many site specifics. Main
design parameters include:
• Solids Loading Rate (SLR) – Thickener diameter and surface area are
determined by the required SLR. Although generally recommended SLRs
are available, WTPs can perform site-specific tests to determine the
optimum design SLR. In general, the coagulant sludge SLR is between 2
and 3 lb/day/ft
2
, and the lime softening sludge SLR is between 20 and 40
lb/day/ft
2
(U.S. EPA, ASCE, AWWA, 1996; AWWA, 2008).
• Hydraulic Loading Rate (HLR) – For WTP sludges, the HLR is not
typically the limiting factor for thickener design (U.S. EPA, ASCE,
AWWA, 1996). However, if large volumes of water will be pumped to the
thickener, equalization may be required. For example, if sedimentation
tanks are emptied periodically, the increased HLR from such a batch
discharge may lead to poor solids removal. HLR is measured in
gal/day/ft
2
.
• Residuals flow – The flow of residuals to the gravity thickener can be
calculated as the summation of the clarified SFBW, sedimentation tank
underflow, and recycle from dewatering.
The cost data sources discussed in this section provide further details on gravity thickener
capacity, and design assumptions.
12-12

Drinking Water Industry Report Section 12 – Treatment Technology Cost Considerations
12.3.4 Sludge Dewatering Centrifuges and Equalization Tanks
The most common type of WTP dewatering is evaporation through non-
mechanical dewatering such as sludge drying beds, ponds, or lagoons, as shown in Table 3-11.
However, these technologies are not universally available, and the feasibility and costs of
lagoons and evaporation ponds depend on land availability. Therefore, EPA analyzed costs for
mechanical dewatering. Specifically, EPA collected information on the costs to install and
operate centrifuges, because this technology can be applied universally to precipitative softening
and conventional filtration residuals treatment. For coagulation plants, WTPs may use belt filter
presses for dewatering; however this application is not common for softening residuals.
Precipitative softening plants may use plate and frame presses for dewatering; however this
application is not commonly used for coagulation residuals due to high operation and
maintenance costs (AWWA, 2008).
As shown in Figure 12-1, thickener sludge is pumped to the dewatering process.
The sludge solids content will fluctuate, and a holding tank for equalization is needed to simplify
the design and operation of dewatering centrifuges.
As with sludge thickening, the dewatering holding tank capacity requirements
depend on SLR. However, these values are less complicated by site specifics than thickener
SLR. The SLR can be calculated based on the influent solids load and holding tank dimensions.
The solids load entering the centrifuges can be calculated as:
Solids Load (lbs/day) = Thickener Sludge (gpd) × Sludge Density (lbs/gal) (Eq. 12-1)
where:
Thickened Sludge = Volume of sludge pumped from the thickener; and
Sludge Density = 65 lb/ft3, or 8.7 lb/gal (AWWA, 2008), by assumption.
The holding tank size will vary based on WTP requirements, such as capacity
limitations. In its 2008 study, the AWWA estimated holding tank diameters between 10 to 100
feet (AWWA, 2008). Once holding tank surface area is determined, the SLR can be calculated
as:
12-13

Drinking Water Industry Report Section 12 – Treatment Technology Cost Considerations
SLR (lbs/day/ft
2
) = Solids Load (lbs/day) ÷ Tank Surface Area (ft
2
) (Eq. 12-2)
The dewatering capacity of centrifuges can then be calculated from the solids
loading rate and dewatering treatment duration. Treatment duration will vary by plant. Small
WTPs may operate the centrifuges for less than eight hours per day, while larger plants may run
multiple shifts daily. For example, treatment duration for a large plant operating two shifts will
be 16 hours per day, or 112 hours per week. Treatment duration for a small plant operating one
five-hour shift will be five hours per day, or only 35 hours per week for a seven-day work week.
12.3.5 Ancillary Equipment
Ancillary equipment includes pumps, piping, instrumentation, biosolids storage
bins, and treatment system housing. In its 2008 study, AWWA estimated the costs for the
ancillary equipment, except pumps, as indirect costs, using a percentage of the total direct capital
cost (AWWA, 2008). Therefore, of the ancillary equipment, only pumps are not included in the
AWWA cost estimate. When sizing and costing a pump, plants review the flow rate required,
hydraulic properties (e.g., need to pump to a higher elevation vs. gravity flow, amount of solids
in the waste stream), any potential corrosion issues, and the need for backup equipment.
In comparison, the EPA WBS cost models estimate the cost of ancillary
equipment, including residuals pumps, piping, instrumentation, storage, and buildings, as direct
line items, based on engineering requirments. In the WBS framework, indirect costs that are not
directly related to the treatment technology used or the amount or quality of the treated water
produced, but that are associated with the construction and installation of a treatment process.
Section 12.4.2 further discusses indirect costs.
12.4 COSTS TO INSTALL AND OPERATE RESIDUALS TREATMENT
SYSTEMS
The cost to install and operate a residuals treatment system includes:
12-14

Drinking Water Industry Report Section 12 – Treatment Technology Cost Considerations
• The capital costs to construct and install treatment units, such as an
equalization tank and centrifuge;
• The indirect capital costs associated with construction and installation,
such as project management;
• Annual costs, such as operations and maintenance requirements; and
• Additional, site-specific costs that vary between WTPs.
Upgrades to or retrofitting of an existing residuals treatment system may require
additional costs that are not included in this section.
12.4.1 Capital Costs for Treatment Units
Estimates of capital costs to construct treatment units are available from sources
including:
• AWWA, 1987: AWWA-developed residuals treatment costs.
• EPA-developed residuals treatment costs (U.S. EPA 1993 and U.S. EPA,
ASCE, and AWWA, 1996).
• AWWA, 2008: AWWA-developed full cost estimates.
• EPA’s forthcoming draft WBS Cost Models.
For certain treatment units, EPA compared estimated treatment unit costs. In
general, the studies are not directly comparable due to differences in methodologies. The 2008
AWWA study built on the earlier two studies, and identified some additional costs that they did
not include: the need for redundancy, differentiation by WTP type (coagulation versus
softening), and trends in solids loads being higher than estimated in the earlier studies. Softening
plants typically have higher costs than conventional filtration plants due to the larger amount of
residuals generated (AWWA, 2008).
The EPA WBS cost models also differentiate by WTP type (including coagulation,
softening, and more than a dozen other technologies) and address the need for redundancy. The
specific capital equipment costs included in a WBS model depend on the WTP technology and
12-15

Drinking Water Industry Report Section 12 – Treatment Technology Cost Considerations
the residuals management option chosen for that technology. The line-item capital costs in the
WBS models for coagulation and softening, however, cover all of the components shown in the
typical treatment train in Figure 12-1. The WBS models also include more ancillary equipment
components as direct line items, instead of indirect costs, and include costs not covered in some
of the previous costing studies, such as land and permitting cost.
12.4.2 Indirect Capital Costs
The magnitude of indirect cost multipliers depends greatly on which cost
components are defined as indirect costs rather than direct capital costs (AWWA, 2008).
Table 12-4 compares the indirect cost factors from the studies.
12.4.3 Annual Operating Costs
Annual operating costs for residuals treatment systems include chemical
purchasing, labor to operate and maintain the treatment units, dewatered sludge disposal,
electricity, and materials to maintain the treatment units. In the AWWA (2008) estimates, sludge
disposal was the most expensive annual cost. AWWA estimated sludge disposal costs from
previously published data. In 2007 dollars, the sludge disposal costs would be $0.37 per wet ton
per mile for transportation and $36.32 per wet cubic ton for disposal (AWWA, 2008). In the
EPA WBS models, the relative magnitude of various operating costs varies depending on a
variety of factors. These factors include, but are not limited to: the WTP technology, the types of
residuals generated, their quantity, the frequency of generation, residuals characteristics, the
types of residuals treatment employed, the disposal or discharge options chosen, and the degree
of automation of the process. Sludge transportation and disposal unit costs, however, are similar
to those in the AWWA (2008) estimates. In 2010 dollars, the WBS unit costs are $0.468 per ton
per mile for transportation and $59.99 per ton for non-hazardous waste disposal.
12.4.4 Additional Costs that Vary Between WTPs
Costs that differ by WTP were excluded from the costs presented in this section.
These costs will vary because of WTP location, receiving stream, and other site-specific factors.
12-16

Drinking Water Industry Report Section 12 – Treatment Technology Cost Considerations
However, they will affect the overall residuals management costs. Additional costs include the
following:
• Sample Collection and Laboratory Analysis. Costs for sample
collection and analysis to determine solids content, free liquids (i.e.,
separate phase), toxicity characteristics, and other parameters. Depending
on the residuals management method selected, sampling requirements
could be minimal or extensive.
• Permits and Other Regulatory Requirements. Costs for permits and
other regulatory requirements. Requirements vary considerably from state
to state and for given management option. Permitting costs vary based on
the capacity and complexity of a unit and the local governing jurisdiction.
Management methods that may require permits include landfills, land
application, evaporation ponds, and storage lagoons. In addition,
generators of hazardous waste are required to comply with EPA Resource
Conservation and Recovery Act (RCRA) generator regulations.
12-17

Drinking Water Industry Report Section 12 – Treatment Technology Cost Considerations
Table 12-4. Indirect Cost Factors and Selected Unit Costs for WTP
Residuals Treatment System Planning
Component
Factor
AWWA
(1986 Cost)
c
EPA
(1992 Cost)
d
AWWA
(2008 Cost)
e
EPA’s Draft WBS Models (2010 Cost)
f
Land
Not estimated as
part of study
$10,000/acre
Not estimated as
part of study
$13,000 to $115,000 per acre (based on system size)
Buildings
$75/ft
2
(1
st
floor);
$50/ft
2
(2
nd
floor)
$33.00/ft
2
Included with
treatment unit cost
$39 to $152/ ft
2
(based on quality; size; and heating, ventilating, and air conditioning
(HVAC))
Piping
A percent of
equipment costs
and experience
from authors
5% of installed
equipment
a
10% of installed
equipment
Included in direct costs. Varies by material, diameter, length, process
size; includes additional length to account for fittings cost
Pipe fittings
20% of piping
costs
b
Electrical
Not estimated as
part of study
1% of installed
equipment
15% of installed
equipment, piping,
and general costs
(see below)
10% of direct cost for outdoor lighting, yard wiring, switchgear,
transformers, and miscellaneous wiring (General building electrical,
such as building wiring and lighting fixtures, is included in the
building cost. Certain other electrical costs are included in direct costs
for system controls and pumps.)
Instrumentation
Not estimated as
part of study
1–2% of installed
equipment
15% of installed
equipment, piping,
and general costs
Technology- and site-specific instrumentation and system controls are
included in direct costs
Engineering fee
Not estimated as
part of study
15% of direct
capital
Included as part of
contractor’s
overhead and
profit
Direct cost multipliers:
20% <1 mgd
12% 1–9.9 mgd
8% >= 10 mgd
Contingency,
bonding, and
mobilization
10% contingency
applied to
manufacturing
furnished
equipment costs.
These costs cover
site-specific
requirements and
extras normally
encountered.
20% of direct
capital
25% of installed
equipment, piping,
general costs,
electrical, and
instrumentation
Contingency: 0% to 13.4% of direct costs
Mobilization/demobilization: 2% to 5% of direct costs
Performance bonds: up to 2.5% of direct costs
12-18

Drinking Water Industry Report Section 12 – Treatment Technology Cost Considerations
Table 12-4. Indirect Cost Factors and Selected Unit Costs for WTP
Residuals Treatment System Planning
Component
Factor
AWWA
(1986 Cost)
c
EPA
(1992 Cost)
d
AWWA
(2008 Cost)
e
EPA’s Draft WBS Models (2010 Cost)
f
Contractor’s
overhead and
profit (non-
construction
cost)
20% of
construction cost
subtotal
12% of direct
capital
30% of total
construction cost
General contractor overhead: 3.2% to 10% of direct costs
(Includes construction management fee and builder’s risk insurance.
Installing contractor overhead and profit is included in direct costs for
installed equipment.)
Additional add-
on costs
Not estimated as
part of study
Not estimated as
part of study
Included as part of
contractor’s
overhead and
profit (non-
construction cost)
Pilot study: equipment rental, analytical costs, labor cost
Permits: vary by technology and site
Additional
indirect cost
categories
Not estimated as
part of study
Not estimated as
part of study
General costs: site
work, yard piping,
and final grading
30% of installed
equipment and
piping
Indirect
construction cost:
30% of installed
equipment, piping,
general costs,
electrical,
instrumentation,
and contingency
(i.e., total direct
construction costs)
Architectural fee: 4.5% to 9% of building costs
Sitework: $10.90/ ft
2
Yard piping: varies by site
Geotechnical: varies by site
Standby power: varies by site
Miscellaneous allowance: 10% of direct costs
Financing during construction: 0% to 5% of direct costs
Legal, fiscal, administrative: 2% of direct costs
a - Piping costs are calculated directly when piping is a significant cost (e.g., for direct discharge).
b - Factor is used when piping costs are calculated directly.
c - AWWA, 1986.
d - EPA, 1993.
e - AWWA, 2008.
f – For updates on the status of the WBS models, please check the EPA webpage at:
http://water.epa.gov/scitech/wastetech/guide/treatment/index.cfm.
12-19

Drinking Water Industry Report Section 12 – Treatment Technology Cost Considerations
• Additional Land Requirements. Some WTPs will require additional land
or have no adequate land adjacent. If land is not available, a residuals
treatment system may not be possible, and alternate scenarios, such as
piping residuals, would be necessary.
• Power Capacity. Depending on location, additional power capacity
needed for residuals treatment may not be available or may require
additional costs.
12.5 REFERENCES
American Water Works Association (AWWA), 1987. Water Treatment Plant Waste
Management. Document Control Number (DCN) DW00186.
AWWA, 2008. Costing Analysis to Support National Drinking Water Treatment Plant Residuals
Management Regulatory Options. Submitted by Environmental Engineering & Technology, Inc.
Newport News, VA. DCN DW03766.
Bosgraaf, 2005. Bosgraaf, Bob (U.S. Filter). Water Treatment Residuals Products Presentation
for EPA. Presentation to EPA on May 25, 2005. DCN DW00156.
Eastern Research Group (ERG), 2006. Presentation: Work Breakdown Structure (WBS)
Residuals Management Cost Model from EPA OGWDW. DCN DW00383.
U.S. Environmental Protection Agency (EPA), 1993. Large Water System Byproducts Treatment
and Disposal Cost Document (EPA 811-D-93-002). Office of Water, Washington, DC. DCN
DW00058.
U.S. EPA, 2006. Identification of Technology Options. Office of Water, Washington, DC.
November 7, 2006. DCN DW03
U.S. EPA, 2007. Review Input Summary: Identification of Technology Options. Office of Water,
Washington, DC. September 2007. DCN DW03750.
U.S EPA, 2009. Technical Analysis for Determination of Technology-Based Permit Limits for
the Guaynabo Drinking Water Treatment Facility NPDES No. PR0022438 (EPA 821-R-11-006).
Office of Water, Washington, DC. March 23, 2009.
U.S. EPA, ASCE, and AWWA, 1996. Technology Transfer Handbook: Management of Water
Treatment Plant Residuals (EPA 625-R-95-008). Office of Research and Development,
Washington, DC. DCN DW03736.
12-20

SECTION 13
ECONOMIC ACHIEVABILITY METHODOLOGY
13.1 INTRODUCTION
This section outlines a methodology for determining whether a proposed residuals
management technology is economically achievable for a public water system (PWS) under their
NPDES permit. Because drinking water systems are usually regulated monopolies or publicly
owned entities, the way to evaluate economic achievability of technological requirements is
different than the methods that would normally be used when evaluating impacts in a
competitive industry.
In a competitive industry, the total cost of installing and operating pollution
control technologies are often not passed on to the consumer. A firm that raises its price in a
competitive industry risks losing sales or even customers. Therefore, companies operating in a
competitive market will usually decide to raise their price by less than the amount of the
additional production cost, lowering their operating profits as a result. In competition, it’s also
possible that the price increase the market will bear would require a firm to lower its operating
profits to the point where they are negative and the firm can’t stay in business.
Because public water systems are regulated monopolies or publicly owned
entities, the rates (prices) they charge their customers are not set in an unregulated competitive
marketplace. Instead, the rates are based on the costs the PWS incurs in delivering water to their
customers. These costs include residuals management expenditures that are incurred as a result
of NPDES permitting, as well as costs associated with complying with Safe Drinking Water Act
regulations. Unless a PWS has access to funds not derived from water services, they are likely to
completely pass on residuals management compliance costs to customers in the form of higher
water rates. Therefore, the ability of the PWS to pay for residuals management technology is
only limited by its ability to raise the water rates it charges its customers. Of course, there is a
limit to how much customers can and will pay for their water services.
13-1

Drinking Water Industry Report Section 13 – Economic Achievability Methodology
The economic analysis of improved residuals management for a PWS is focused
on the impact of water rate increases on customers and, in particular, on their residential
customers. Pollution abatement measures would be deemed economically achievable if the
PWS’s customer base is able to bear the impact of the water rate increases associated with the
costs of the residuals management improvements. The analysis of customer impact could be
extended to businesses; however, the impact to businesses of water rate increases is not likely to
be substantial, except potentially in instances where effluent treatment costs for the PWS are
very high and certain business customers rely heavily on publicly supplied water as an important
factor in their production processes. But even these firms may be able to pass costs on to their
customers.
13.2 A METHODOLOGY FOR DETERMINING THE ECONOMIC
ACHIEVABILITY OF BEST PROFESSIONAL JUDGMENT EFFLUENT
LIMITATIONS FOR A PUBLIC WATER SYSTEM
The approach to determining the economic achievability of residuals management
technology improvements is conducted at the system or utility level, depending on whether the
costs for a treatment technology would be spread amongst consumers at the system level or
across all the customers of the larger utility. For the purpose of determining the financial strength
of the larger corporate entity which owns the individual drinking water treatment plant
implementing the NPDES residuals technology improvements and the impacts to the large
corporate entity’s customer base, EPA must look to the level of the system (the PWS). It is at the
system level that the costs of technology improvements are financed, and it is the system that can
spread the costs of upgrades to a specific plant or plants across its total customer base. In some
instances a larger utility may own more than one system and spreads the cost of technology
improvements across those systems. In these cases the proper level of financial assessment is at
the level of the utility.
The assessment of economic achievability for NPDES residuals management
technology at the PWS level consists of four steps, once the annualized costs associated with the
technology improvements have been determined:
1. Estimate the increase in water rates for household customers.
13-2

Drinking Water Industry Report Section 13 – Economic Achievability Methodology
2. Estimate the increase in the annual cost of water as a result of residuals
management improvements for household customers by Census-based
income class.
3. Based on the ratio of water cost increase as a result of additional residuals
handling expenditures to household income by Census-based income
class, estimate the number and percentage of households (total and within
income classes) for which the estimated increase in household cost of
water exceeds the chosen percent of median household income
achievability threshold.
4. For water systems in which the number and/or percentage of adversely
affected households exceed the relevant threshold, assess the potential for
using rate-structure-based methods to shift the potential water rate
increase away from households for which the increase is determined to be
too great.
Embedded within these four steps are two important threshold criteria that are not
prescribed here. The first criterion is the percent of median household income achievability
threshold which represents the maximum acceptable portion of household income that could be
expended on new residuals management treatment technologies without significantly affecting a
household’s financial condition. It is important to remember that, this threshold value represents
that fraction of income which can be spent by the household on the incremental cost of the new
NPDES permitting requirements. The second criterion to be set is the number of households
whose cost share is greater than the threshold percent of income that would cause the technology
costs to be considered not economically achievable. Ultimately, which percent of income
achievability threshold used, as well as the number of households for which rates exceed the
percent of income threshold, are important policy decisions for the Director to consider.
In order to provide a numerical example of this suggested NPDES residuals
management economic achievability methodology, EPA selected a median annual household
income threshold. This threshold is based on a review of the economic support documents from
past Effluent Limitation Guideline (ELG) rulemakings. Particular attention was focused on the
regulated entities that operate as a local monopoly much the same way as PWSs operate.
Through this review, EPA found that the economic achievability analysis that most closely
mirrored the proposed drinking water NPDES permit cost achievability methodology was
conducted for the final “Effluent Limitations Guidelines, Pretreatment Standards, and New
13-3

Drinking Water Industry Report Section 13 – Economic Achievability Methodology
Source Performance Standards for the Landfills Point Source Category” rulemaking. The
Landfills ELG economic achievability analysis used a compliance cost share of household
income test. This test was used to assess community level economic impacts when municipally-
owned landfills would likely pass costs on to household customers. The ratio of the average per
household share of compliance costs to median household income was calculated and if the ratio
exceeded a 1.0 percent threshold EPA determined that the technology costs would likely have a
“severe impact” to the community. Although the achievability methodology presented here is
more refined, the 1.0 percent of median household income threshold value from the Landfills
ELG will be utilized in the example analysis. Because the 1.0 percent threshold in the Landfills
ELG signified a high probability of severe economic impacts to the community EPA
recommends to the Director that the percent of median household income ultimately selected be
lowered if moderate impacts are the measure of interest.
EPA did not select a value for the maximum number of households being served
by the PWS that would be allowed to receive a treatment cost share greater than the threshold
percent of income in the following example. The Agency does want to note that: (1) a decrease
in the allowed percentage of households whose share of costs exceeds the household income
threshold (or a decrease in the income threshold) will make the economic achievability more
stringent; and (2) increasing the number of households that can exceed the income threshold (or
raising the income threshold value) will have the effect of making the achievability test less
stringent.
The remainder of this section demonstrates how to go about completing the four
steps to determining the economic achievability of NPDES residuals management technology
improvements at PWSs.
13.2.1 Estimate Increase in Water Rates to Household Customers
Regardless of the specific criteria used to determine economic achievability for
PWSs, the analysis should begin with estimating the increase in total water costs to all
households in the water system service territory due to the proposed improvements. This figure
represents the total revenue to be raised by the increase in water rates to household customers,
13-4

Drinking Water Industry Report Section 13 – Economic Achievability Methodology
and the estimated quantity of water consumed by households. Estimating the change in water
rates involves two main steps:
1. Estimate the aggregate rate effect due to technological improvements; and
2. Estimate the change in water rates per unit of consumption, by customer
class.
13.2.1.1 Estimate Total Rate Effect of Compliance Costs
The estimated change in water rates, and resulting costs to households, should
reflect how the cost to adopt technology improvements would actually be incorporated into a
PWS’s rate structure. The change in a PWS’s revenue requirements is typically the basis for
setting water rates. For annually recurring costs (e.g., operation and maintenance (O&M) costs),
this analysis is straightforward: such costs are simply added to the system’s total revenue
requirements. However, for capital or other non-annually recurring costs, completing this
analysis will require several assumptions.
The first assumption involves how these costs would be financed, including the
cost and terms of the financing. Funds may be borrowed, taken from current operating revenue,
or, if the company is privately owned, gained from issuing equity stakes in the company. The
recommended assumption is to use the weighted average of the reported cost of capital and
repayment periods for projects undertaken within the past five years to establish the cost and
terms of the capital required.
The second assumption involves how costs would be incorporated into the PWS’s
near-term rate structure. This issue includes the cost recovery and rate-making practices at the
affected PWS. The cost recovery for capital outlays may be:
• Fixed to a constant annual value over the cost recovery period, or
• Based on a framework of depreciating rate base with an allowed rate of
return.
13-5

Drinking Water Industry Report Section 13 – Economic Achievability Methodology
Under the constant annual payment framework, the cost analysis is relatively
straightforward. The annual charge for capital outlays is calculated as a constant annual payment,
based on an interest rate
32
and repayment term of the amount to be financed. This approach is
appropriate if the average repayment period is approximately equal to the estimated useful life of
the capital improvements. The annual charge would be calculated as follows:
( )
( )
1r1
r1r
Outlay Capital
Charge
Capital
N
N
−+
+×
×=
(Eqn. 13-1)
where:
Capital charge = The constant annual rate increase to recover the new
technology capital outlay over the N year capital recovery
period at the interest rate r.
Capital outlay = The capital outlay for implementing the new technology (or
other non-annually occurring outlays associated with the
new technology).
N = The number of years over which the Capital outlay is
recovered in water system rates presumed to be equal to the
estimated useful life of the new capital equipment.
r = The allowed interest rate for recovering capital outlay over
the capital recovery period, presumed to be equal to the
average of interest rates reported for recent borrowings by
the water system.
The situation of a depreciating rate base with allowed rate of return follows the
conventional regulated utility ratemaking framework, but the cost analysis is somewhat more
complicated. The annual charge is based on the amount of capital outlay placed into “rate-
base,”
33
the depreciation period for the capital outlay, and the allowed rate of return on the rate-
base. The capital charge in any year is typically calculated as the sum of the straight-line
depreciation of the initial rate-base value and the product of the rate of return and the depreciated
rate-base value. Under this approach, the annual capital charge would be calculated as follows:
32
The interest rate should correspond to the credit ratings of the PWS. The bond yield for the appropriate credit
rating can be found in sources such as S&P or Moody’s Investor Services.
33
“Rate-base” refers to the aggregate value of capital the PWS is entitled to recover through customer water rates,
with a rate of return.
13-6

Drinking Water Industry Report Section 13 – Economic Achievability Methodology
( )
( )
(
)
1
n
N/
i
-N
Outlay CapitalrNOutlay/
Capital
Charge
Capital
n
0i
+
××+
=
∑
=
(Eq. 13-2)
where:
Capital Charge = The average amount of the technology capital outlay
recovered annually in the total water rate over the first
(n+1) years of capital recovery. For this analysis, EPA
would propose to look at a relatively short period of initial
rate effect – e.g., the first three years, in which case the
value n would be 2.
Capital Outlay = The capital outlay for implementing the new technology (or
other non-annually recurring outlay associated with the
new technology).
N = The number of years over which the Capital Outlay is
depreciated for ratemaking purposes – presumed to be
equal to the estimated useful life of the new capital
equipment.
i = The number of years since placing the Capital Outlay into
rate base.
n+1 = The total number of years for which the annual charge is
calculated. For this analysis, EPA would use something like
n+1=3, or n=2.
r = The allowed rate of return on rate base – presumed to be
equal to the average of interest rates reported for recent
borrowings by the water system.
Finally, the sum of the annual recurring costs and the charges for capital outlays
for compliance with the proposed abatement technology yields the total increase in annual water
rates resulting from the new technology:
Total Rate Increase = Recurring Costs + Capital Charge (Eq. 13-3)
where:
Total Rate increase = The annual increase in total water system rates resulting
from implementing new technology.
Recurring Costs = Technology costs that recur annually – e.g., recurring
operating and maintenance expenses.
Capital Charge = Annual recovery of the capital outlay for new technology.
13-7

Drinking Water Industry Report Section 13 – Economic Achievability Methodology
13.2.1.2 Estimate Rate Effect per Unit of Water Consumed
The total rate increase must be allocated to the different types of customers the
PWS serves. As above, this analysis may require different treatments for the recurring cost and
capital cost components.
The recurring cost component is assumed to be the same for all customer classes.
(This would not be true if pretreatment contributed to technology costs, as pretreatment
requirements are derived from certain customer classes). The recurring cost rate effect is
calculated by dividing the annual recurring costs charge by the total volume of finished and
partially treated
34
water sold annually by the PWS.
ΔRate
RecurringCosts
= Recurring Costs ÷ Treated Volume (Eq. 13-4)
where:
ΔRate
RecurringCosts
= Increase in water system rates, per-unit-consumed, from the
recurring cost component of new technology (assume rate
structure is preserved and increase is across-the-board).
Recurring Costs = Total rate increase from costs the recur annually.
Treated Volume = Total volume of finished and partially treated water sold
annually.
As in subsection 13.2.1.1, the capital charge component presents a potentially
more challenging case as the capital charge is more likely to be allocated differentially by
customer class than the recurring cost component of the water rate charge. For example, if the
amount of capital equipment required for compliance with the effluent limitation is determined
by the peak water demand during a several-hour period of the day, then it would be reasonable to
allocate capital costs according to the contribution of individual customer classes to demand
levels during different periods (e.g., user profile during low-demand, medium-demand, and high-
demand periods). Because PWS-specific rate structure information may not be available, and in
order to be conservative in this achievability analysis, the capital charge should be allocated to
the household rate class based on the greater of:
34
The Agency suggests including water that is treated at any level – i.e., both finished water and partially treated
water – in the denominator for calculating the unit rate increases. The logic for this definition of the denominator is
that the treatment requirements, and thus cost, will apply to the residuals of water treatment, whether for finished
water or partially treated water.
13-8

Drinking Water Industry Report Section 13 – Economic Achievability Methodology
1. The percentage of total water consumed by residential customers, or
2. The percentage of total water sales revenue from residential customers.
Once a component of capital costs has been allocated to the residential class, the
average rate impact can be calculated based on the total water volume sold to residential
customers.
( )
[ ]
res
resres
ChargeCapital
VolumeTreated
ChargeCapitalShareRev,ShareWaterMax
Rate
×
=∆
(Eq. 13-5)
where:
ΔRate
Capital Charge
= Increase in water system rates to residential customers, per-
unit-consumed, from the capital charge component of new
technology costs
Water Share
res
= Residential customers’ share of total water consumption
Rev Share
res
= Residential customers’ share of total water sales revenue
Capital Charge = Total annual rate increase from the new technology capital
outlay
Treated Volume
res
= Total volume of finished water sold annually to residential
customers, in same units as rate structure is expressed
Summing the Recurring Cost and Capital Charge rate components yields the total
per-unit-consumed rate increase to residential customers:
ΔRate
Total
= ΔRate
Recurring Costs
+ ΔRate
Capital Charge
(Eq. 13-6)
where:
Δ Rate
Total
= Total increase in water system rates to residential
customers, per-unit-consumed, resulting from new
technology
Δ Rate
Recurring Costs
= Increase in water system rates, per-unit-consumed, from the
recurring cost component of new technology costs
Δ Rate
Capital Charge
= Increase in water system rates to residential customers, per-
unit-consumed, from the capital charge component of new
technology costs
13-9

Drinking Water Industry Report Section 13 – Economic Achievability Methodology
13.2.2 Estimate Increase in Annual Water Service Cost for Household Customers
The next step in the analysis is to calculate the increase in annual water service
cost to households. This calculation involves multiplying the unit rate increase, from the
preceding step, by the estimated average household water consumption. This general
understanding can be more accurately described within the framework of the following two sub-
steps:
1. Estimate the average quantity of water consumed per household; and,
2. Estimate the annual increase in household water service cost.
13.2.2.1 Estimate Average Household Water Consumption
Average household water consumption is calculated by dividing total water
quantity supplied to residential customers by the estimated number of households served.
35
The
number of households served by a PWS is estimated by dividing the reported number of people
served by the system – available through EPA’s Safe Drinking Water Information System
(SDWIS) – by the average number of persons per household within the service area of the PWS.
The average number of persons per household is calculated from Census data
36
for the ZIP codes
reported as served by the PWS. Following is the calculation for the number of households served
by a water system:
37
35
The calculation of the number of households served is required because many PWSs do not know the number of
households served, but rather, know the number of residential connections served. Since many multi-family
dwellings (e.g., apartment buildings) do not have separate meters for each household, the number of residential
connections will likely underestimate the number of households served. However, multi-family dwellings may not
see 100% cost pass-through, due to some units being billed for water as part of rent, which is subject to market
forces.
36
Based on the most recently available Census data.
37
In this equation, the average number of households is calculated by using the average household size by ZIP code
(or county) and weighting by number of households reported in the ZIP code (or county), both as reported by the
Census, instead of simply summing total population and households over the ZIP codes and dividing total
population by total households. This calculation is necessary because the reported average household size for a ZIP
code (or county) as reported in the Census frequently differs from the average household size that would be
calculated for a ZIP code (or county) using the reported population and households for a ZIP code (or county).
13-10

Drinking Water Industry Report Section 13 – Economic Achievability Methodology
×
=
∑
∑
=
=
Z
1z
z
Z
1z
zz
est
CensusHHSN
)CensusSizeHHSCensusHHS(N
Persons
HouseholdsN
(Eq. 13-7)
where:
N Households
est
= Estimated number of households served currently by the
PWS.
Persons = Number of persons served by the PWS.
Z = The total number of zip codes (or counties) served by the
PWS.
z = Zip Code or county index.
N HHS Census
z
= Number of households reported in Census data for zip code
(or county), z.
HHS Size Census
z
= Average household size reported in Census data for zip
code (or county), z.
To calculate annual water consumption per average household, divide the annual
flow of water to residential customers (available from the PWSs) by the number of households
served by the water system:
est
lresidentia
av
HouseholdsN
VolWater
ConsWaterHH =
(Eq. 13-8)
where:
HH Water Cons
av
= Annual water consumption for the average household.
Water Vol
residential
= Total water volume delivered annually to residential
customers.
N Households
est
= Estimated number of households served currently by the
PWS.
13.2.2.2 Estimate Increase in Annual Water Service Cost for Household Customers,
Based on Estimated Household Water Consumption
The water consumption quantity is then multiplied by the estimated change in per
unit water rates to calculate the increase in annual water cost to household customers:
ΔWater Cost
av hh
= HH Water Cons
av
+ ΔRate
Total
(Eq. 13-9)
13-11

Drinking Water Industry Report Section 13 – Economic Achievability Methodology
where:
Δ Water Cost
av hh
= Increase in water service cost to the average household,
resulting from new technology.
HH Water Cons
av
= Annual water consumption for the average household.
Δ Rate
Total
= Total increase in water system rates to residential
customers, per-unit-consumed, resulting from new
technology.
13.2.3 Estimate Number and Percentage of Households, by Water System, for
which the Annual Household Water Service Cost Increase Exceeds a Percent
of Income Achievability Threshold
As the initial test of economic achievability for residuals treatment under the
NPDES program, calculate the number of households for which the estimated increase in water
service cost will exceed the chosen income achievability threshold. As noted previously, the
income achievability threshold used, as well as the maximum number of households being
served by the PWS that would be allowed to receive a treatment cost share greater than the
percent of income threshold before the proposed new NPDES treatment technology would be
considered not economically achievable, are important decisions for the Director. The example
threshold presented is 1.0% of median household income.
A household is counted as facing an achievability challenge at a given threshold if
the ratio of the estimated water cost increase to household income exceeds the threshold.
Implementing this achievability test concept requires several additional steps, as described
below.
13.2.3.1 Adjust for the Difference in the Reporting Year of the Household Income
Information for the Most Recent Census
The difference in the reporting year of the household income information should
be adjusted for the most recent Census and the year for which new technology costs will be
estimated. To compare the increase in water service cost with household income at current
levels, the household counts by income range from the most recent Census need to be brought
forward to the current year. Several issues arise in this adjustment:
13-12

Drinking Water Industry Report Section 13 – Economic Achievability Methodology
• General change in household income over time;
• Shifts in household counts within the income distribution; and
• Change in the aggregate number of persons and households over time.
The decennial Census reports the number of households in specific income
ranges. Below $50,000 household income, the household counts are reported in $5,000 ranges
(with the exception of the first income range, which includes $0-$9,999). Above $50,000, the
income ranges widen progressively, from $10,000 to $50,000, and finally ending at “greater than
$200,000.” Although the Census publishes a variety of sample-based updates between the
decennial census years, it does not publish an update of the data on household count by income
range. Table 13-1 provides an example of the income distribution information provided from the
2000 Census.
Table 13-1. Example of Income Distribution from the 2000 U.S. Census
Income Range
Number of Households
0
to
9,999
1,056
10,000
to
14,999
1,311
15,000
to
19,999
1,523
20,000
to
24,999
1,708
25,000
to
29,999
2,014
30,000
to
34,999
3,003
35,000
to
39,999
2,322
40,000
to
44,999
1,307
45,000
to
49,999
2,636
50,000
to
59,999
4,659
60,000
to
74,999
2,839
75,000
to
99,999
4,682
100,000
to
124,999
3,396
125,000
to
149,999
1,908
150,000
to
199,999
1,452
200,000
and
Above
1,299
A two-step process is used to adjust the households-by-income-range data from
the census year to the present (or as close to the present as is possible based on U.S. Census
reporting). First, the income-range values from the census year are adjusted to the present year
based on the change in median household income by state (or possibly county) as reported in the
non-decennial series published in the American Community Survey and the Annual Social and
13-13

Drinking Water Industry Report Section 13 – Economic Achievability Methodology
Economic Supplement (U.S. Census). This adjustment holds as constant the household counts by
income range from the census year data, but shifts upward (or downward) the definition of the
ranges to which the household counts apply based on the change in income at the 50
th
percentile
of the household income distribution (see calculation in the equation below). As an example,
Table 13-2 shows the income range limits from Table 13-1 adjusted for a 10 percent increase in
household income. All that is done is the low-end and high-end of each income range is
multiplied by 1.10. The number of households in each range remains constant for now.
Income Range
t,l
= (1+ΔMHI
l
) × Income Range
Census
(Eq. 13-10)
where:
Income Range
t,l
= Income range value after adjustment to the present (time t)
for location l (state or county)
Δ MHI
l
= Percent change in median household income from
decennial census (e.g. 2000) to time t for location l (state or
county)
Income Range
Census
= Income range value used in Census household-by-income
level reports – e.g., $10,000.
Table 13-2. Example of Income Distribution Provided by the U.S. Census
With Ranges Updated to Current Year (10% increase in income)
Income Range
Number of Households
0
to
10,999
1,056
11,000
to
16,499
1,311
16,500
to
21,999
1,523
22,000
to
27,499
1,708
27,500
to
32,999
2,014
33,000
to
38,499
3,003
38,500
to
43,999
2,322
44,000
to
49,499
1,307
49,500
to
54,999
2,636
55,000
to
65,999
4,659
66,000
to
82,499
2,839
82,500
to
109,999
4,682
110,000
to
137,499
3,396
137,500
to
164,999
1,908
165,000
to
219,999
1,452
220,000
and
Above
1,299
13-14

Drinking Water Industry Report Section 13 – Economic Achievability Methodology
The second step is to adjust the number of households in the Census distribution –
both in total and within the income ranges – according to the ratio of the total population
currently served (again, as reported in SDWIS), to total population for the identified ZIP codes
(or counties) as reported in the Census. This adjustment accounts for both the change in total
population since the census year and for the population coverage differential resulting from only
a part of the ZIP codes (or counties) reported by the drinking water system actually being served
by the system (see calculation in equation below). As an example, Table 13-3 shows the number
of households from Table 13-2 adjusted for a 3 percent increase in population. So, the number of
households from Table 13-2 is multiplied by 1.03 for each income range.
N Households
ir,t
= (Persons Served
t
÷ Population
Census
) × N Households
ir,Census
(Eq. 13-11)
where:
N Households
ir,t
= Number of households in Income Range ir at time t.
Persons Served
t
= Number of persons reported served by the PWS at time t.
Population
Census
= Total population reported at time of Census data for zip
codes or counties served by the PWS.
N Households
ir,Census
= Number of households in Income Range ir as reported at
time of Census data.
Table 13-3. Example of Income Distribution Provided by the U.S. Census
With Ranges and Number of Households Updated to Current Year (10%
increase in income and 3% increase in population)
Income Range
Number of Households
0
to
10,999
1,088
11,000
to
16,499
1,350
16,500
to
21,999
1,569
22,000
to
27,499
1,759
27,500
to
32,999
2,074
33,000
to
38,499
3,093
38,500
to
43,999
2,392
44,000
to
49,499
1,346
49,500
to
54,999
2,715
55,000
to
65,999
4,799
66,000
to
82,499
2,924
82,500
to
109,999
4,822
110,000
to
137,499
3,498
137,500
to
164,999
1,965
165,000
to
219,999
1,496
220,000
and
Above
1,338
13-15

Drinking Water Industry Report Section 13 – Economic Achievability Methodology
13.2.3.2 Accounting for the Lack of Information on How Household Income is
Distributed within the Census-Reported Income Ranges
The Census provides the number of households by the income ranges described
above. In this analysis, the objective is to calculate the number of households for which the
estimated increase in water service cost exceeds a threshold percentage of household income. For
each PWS, the household income level at which the estimated increase in water service cost
equals a threshold percentage is determined using on the equation below.
Inc
threshold level,i
= ΔWater Cost
av hh
÷ Income Threshold
i
(Eq. 13-12)
where:
Inc
threshold level, i
= Threshold income level, based on income threshold i.
Δ Water Cost
av hh
= Increase in water service cost to the average household,
resulting from compliance.
Income Threshold = Threshold percentage of income the compliance costs
cannot exceed i.
This step is followed by the estimation of the number of households served by the
PWS with household income less than that threshold income level. In all likelihood, a threshold
income level will fall within, and not at the edge of, a Census income range. Accordingly, the
fraction of households within a Census income range that fall below a threshold income level
must be estimated. For simplicity’s sake, assume that households are uniformly distributed over
the income values within an income range. As a result, the fractional point at which the threshold
income level lies within an income range will also be the fraction of households within that
income range that fall below the threshold level. Of course, all households in an income range
that is below the range in which the threshold income level falls will be below the threshold
income level.
38
38
The assumption of a uniform distribution of income within each income range inevitably involves error and could
overstate or understate the fraction of households within an income range that fall below an impact threshold.
Nevertheless, the assumption of a uniform distribution within an income range is a reasonable approach. In applying
the uniform-distribution assumption, the Agency warns about the potential for overestimation of adverse impact in
the lowest income range segment – less than $10,000 (before adjusting for income change over time) – if that range
includes a threshold impact income value.
13-16

Drinking Water Industry Report Section 13 – Economic Achievability Methodology
The occurrence of households for which the water service cost increase exceeds a
threshold income level is calculated as follows:
(
)
(
)
*
ir
mn
ir*,
mxir*,
mnir*,level threshold
incir*,
Households N
IncInc
IncInc
Households
N ×
−
−
=
(Eq. 13-13)
where:
N Households
ir*, inc
= Number of households in Income Range ir *with income
below threshold income level (inc), where Income Range
ir* contains the threshold income level inc.
Inc
threshold level
= Threshold income level, calculated above.
Inc
ir*, mn
= Minimum value of Income Range ir*.
Inc
ir*, mx
= Maximum value of Income Range ir*.
N Households
ir
= Total number of households in Income Range ir* estimated
served by the PWS.
Lastly, the total number of households with income below the threshold income
level is aggregated over all income ranges.
∑
−
=
+=
1*ir
1ir
irincir*,inc
HouseholdsNHouseholdsNHouseholdsN
(Eq. 13-14)
where:
N Households
inc
= Number of households over all income ranges with income
below threshold income level (inc).
N Households
ir*, inc
= Number of households in Income Range ir* with income
below threshold income level (inc), where Income Range
ir* contains the threshold income level inc.
N Households
ir
= Number of households in Income Ranges ir below Income
Range ir*.
Table 13-4 follows from the examples above. This table shows the number of
households (and the percentage of households) that are expected to realize an increase in water
costs higher than the achievability income threshold (1.0 percent of household income). In this
example, assume the cost of compliance is $6.69 million, or $175.00 per household. The
threshold income level is then $17,500 which falls in the adjusted Census income range of
$16,500 - $21,999 (the shaded row in Table 13-4). The number of households in the Census
income range where the increase in water cost has an impact greater than the achievability
income threshold is calculated to be 285. The number of households above the achievability
13-17

Drinking Water Industry Report Section 13 – Economic Achievability Methodology
threshold in lower income ranges is always equal to the number of households in the range,
while the number of households above the achievability threshold in higher income ranges is
always equal to zero. The total number of households above the achievability income threshold
is 2723, which equals 7 percent of the total households served by the PWS.
Table 13-4. Example of the Calculation of Number and Percent of Households above an
Achievability Threshold (1.0% of Median Household Income)
Income Range
Number of
Households
Compliance
Costs Per
Household
Achievability
Threshold
Threshold
Income
Level
Number of
Households
above
Achievability
Threshold
Percent of
Households
above
Achievability
Threshold
0
to
10,999
1,088
$175.00
1.0%
$17,500
1,088
100%
11,000
to
16,499
1,350
$175.00
1.0%
$17,500
1,350
100%
16,500
to
21,999
1,569
$175.00
1.0%
$17,500
285
18%
22,000
to
27,499
1,759
$175.00
1.0%
$17,500
0
0%
27,500
to
32,999
2,074
$175.00
1.0%
$17,500
0
0%
33,000
to
38,499
3,093
$175.00
1.0%
$17,500
0
0%
38,500
to
43,999
2,392
$175.00
1.0%
$17,500
0
0%
44,000
to
49,499
1,346
$175.00
1.0%
$17,500
0
0%
49,500
to
54,999
2,715
$175.00
1.0%
$17,500
0
0%
55,000
to
65,999
4,799
$175.00
1.0%
$17,500
0
0%
66,000
to
82,499
2,924
$175.00
1.0%
$17,500
0
0%
82,500
to
109,999
4,822
$175.00
1.0%
$17,500
0
0%
110,000
to
137,499
3,498
$175.00
1.0%
$17,500
0
0%
137,500
to
164,999
1,965
$175.00
1.0%
$17,500
0
0%
165,000
to
219,999
1,496
$175.00
1.0%
$17,500
0
0%
220,000
and
above
1,338
$175.00
1.0%
$17,500
0
0%
Total
38,228
2,723
7%
13.2.3.3 Determining Public Water System-Level Achievability Income Thresholds
Once the number (and percentage) of households in a service territory for which
the estimated increase in water service cost would exceed an achievability income threshold is
calculated, the Director still has to determine if these numbers constitute an economically
achievable solution for the PWS as a whole. As mentioned above, this important question is
subjective and a policy decision that must be made by the permitting authority.
13-18

Drinking Water Industry Report Section 13 – Economic Achievability Methodology
For example, if the permitting authority believes that 1.0 percent of household
income is the correct achievability threshold, 2,723 or seven percent of the households served by
the PWS would have difficulty paying for the higher cost of water associated with the
compliance cost of the permit limitations.
13.2.4 Assessing the Impact of Rate Structure on the Achievability Determination
The example above assumes that all households served by the PWS share equally
in the additional costs associated with compliance, which is not necessarily the case. A
community and its PWS may be able to shift costs away from more economically vulnerable
population segments via increasing block rates, lifeline rates or other income support
mechanisms. These rate structures and programs should be considered when conducting an
achievability analysis. Table 13-5 provides an example of how the achievability analysis can be
modified to take into account a simplistic lifeline-type rate structure. In this case, the cost of
compliance is the same as in the example above ($6.69 million). However, in this example,
because of a lifeline rate structure, no household with annual income below $16,500 will incur
any additional rate increase. Therefore, the cost of compliance is shared among the remaining
households at a greater rate ($186.92 per household versus $175.00 in the earlier example). In
this case, the threshold income level rises to $18,692, but at the same time, fewer households
exceed the achievability threshold (two percent versus seven percent in the example above). The
lifeline rate structure partially mitigates the achievability concerns of this effluent limitation. Of
course, the permit authority still has to decide if the lifeline rate structure mitigates the
achievability issue enough to determine that the effluent limitations are economically achievable.
If a PWS has an increasing block rate structure, they could choose to only
increase the highest end of the block rates. This would pass the entire increase in cost to
households that are consuming the largest quantities of water and are most likely those
households that can best afford a rate increase.
13-19

Drinking Water Industry Report Section 13 – Economic Achievability Methodology
Table 13-5. Example of the Calculation of Number and Percent of Households above an
Achievability Threshold (1.0% of Median Household Income) assuming a Lifeline Rate
Structure for Income Below $16,500
Income Range
Number of
Households
Compliance
Costs Per
Household
Achievability
Threshold
Threshold
Income
Level
Number of
Households
above
Achievability
Threshold
Percent of
Households
above
Achievability
Threshold
0
to
10,999
1,088
$0
1.0%
$0
0
0%
11,000
to
16,499
1,350
$0
1.0%
$0
0
0%
16,500
to
21,999
1,569
$186.92
1.0%
$18,692
628
40%
22,000
to
27,499
1,759
$186.92
1.0%
$18,692
0
0%
27,500
to
32,999
2,074
$186.92
1.0%
$18,692
0
0%
33,000
to
38,499
3,093
$186.92
1.0%
$18,692
0
0%
38,500
to
43,999
2,392
$186.92
1.0%
$18,692
0
0%
44,000
to
49,499
1,346
$186.92
1.0%
$18,692
0
0%
49,500
to
54,999
2,715
$186.92
1.0%
$18,692
0
0%
55,000
to
65,999
4,799
$186.92
1.0%
$18,692
0
0%
66,000
to
82,499
2,924
$186.92
1.0%
$18,692
0
0%
82,500
to
109,999
4,822
$186.92
1.0%
$18,692
0
0%
110,000
to
137,499
3,498
$186.92
1.0%
$18,692
0
0%
137,500
to
164,999
1,965
$186.92
1.0%
$18,692
0
0%
165,000
to
219,999
1,496
$186.92
1.0%
$18,692
0
0%
220,000
and
Above
1,338
$186.92
1.0%
$18,692
0
0%
Total
38,228
628
2%
13.3 REFERENCES
Dalhuisen, Jasper M., Raymond J. G. M. Florax, Henri L. F. de Groot, and Peter Nijamp. 2003.
“Price and Income Elasticities of Residential Water Demand: A Meta-Analysis.” Land
Economics 79(2): 292-308.
Dalhuisen, Jasper M., Raymond J. G. M. Florax, Henri L. F. de Groot, and Peter Nijamp. 2001.
“Price and Income Elasticities of Residential Water Demand: Why empirical estimates differ.”
Tinbergen Institute Discussion Paper. Department of Spatial Economics, Vrije Universiteit and
Tinbergen Institute, Amsterdam, The Netherlands.
Environmental Finance Center. Rate Setting Analysis: Public Water Supply Financial Capacity,
Developing Water Rates. No date. http://www.maxwell.syr.edu/efc/RateSettingAnalysis.htm.
Environmental Finance Center, Syracuse, NY.
13-20

Drinking Water Industry Report Section 13 – Economic Achievability Methodology
Environmental Finance Center at Boise State University. 2002. Drinking Water System
Management Handbook: Administration of a drinking water system through financial, technical
and managerial planning.
http://sspa.boisestate.edu/efc/Publications/Drinking%20Water%20System%20Management%20
Handbook.PDF. Environmental Finance Center at Boise State University, Boise, ID. February
2002.
Midwest Assistance Program, Inc. 1999. North Dakota Small Community Water System’s
Handbook on Developing and Setting Water Rates. http://www.map-
inc.org/Publications/Publications/WatrRate.pdf. Midwest Assistance Program, Inc. New Prague,
MN.
Rubin, Scott J. 2001. Criteria to Assess the Affordability of Water Service. National Rural Water
Association, Rural Water Partnership Fund White Paper. October, 2001.
Rubin, Scott J. 2002. Criteria to Assess the Affordability Concerns in Conference Report for
H.R. 2620. National Rural Water Association, Rural Water Partnership Fund White Paper.
January, 2002.
U.S. Environmental Protection Agency. 1995a. Interim Economic Guidance for Water Quality
Standards. Workbook, Office of Water. EPA-823-B-95-002, March 1995.
U.S. Environmental Protection Agency. 1998a. National-Level Affordability Criteria Under the
1996 Amendments to the Safe Drinking Water Act. Prepared by International Consultants, Inc.,
Hagler Bailly Services, Inc and Janice A. Beecher, Ph.D. for the EPA. August, 1998.
U.S. Environmental Protection Agency. 1998b. Information for States on Developing
Affordability Criteria for Drinking Water. Office of Water. EPA 816-R-98-002, February, 1998.
U.S. Environmental Protection Agency. 1999a. Economic Analysis of Final Effluent Limitations
Guidelines and Standards for the Landfills Point Source Category. Office of Water. EPA-821-B-
99-005, November 1999.
U.S. Environmental Protection Agency. 1999b. Revised Interim Guidance for EPA Rulewriters:
Regulatory Flexibility Act as Amended by the Small Business Regulatory Enforcement Fairness
Act, Office of Regulatory Management and Information. March 29, 1999.
U.S. Environmental Protection Agency. 2000a. Arsenic in Drinking Water Rule Economic
Analysis. Prepared by Abt Associates Inc. for Office of Ground Water and Drinking Water,
EPA-815-R-00-026, December.
U.S. Environmental Protection Agency. 2000b. Economic Analysis of the Radionuclides
National Primary Drinking Water Regulations. Prepared by Industrial Economics, Inc. for Office
of Ground Water and Drinking Water. December, 2000.
U.S. Environmental Protection Agency. 2000c. Guidelines for Preparing Economic Analyses,
September, 2000.
13-21

Drinking Water Industry Report Section 13 – Economic Achievability Methodology
U.S. Environmental Protection Agency. 2002. Affordability Criteria for Small Drinking Water
Systems: An EPA Science Advisory Board Report. EPA-SAB-EEAC-03-004, December 2002.
U.S. Environmental Protection Agency. 2005. Economic Analysis for the Final Stage 2
Disinfectants and Disinfection Byproducts Rule. Office of Water, EPA-815-R-05-010, December
2005.
13-22

SECTION 14
GLOSSARY, ACRONYMS, AND ABBREVIATIONS
A
Activated carbon – Carbon particles usually obtained by carbonization of cellulosic material in
the absence of air and possessing a high adsorptive capacity. Process is to typically heat carbon
to increase porosity surface area.
Administrator – The Administrator of the U.S. Environmental Protection Agency.
Adsorption – The adherence of a gas, liquid, or dissolved material to the surface of a solid.
Aeration – Process that mixes air and water, normally by injecting air into water, spraying water
into the air, or allowing water to pass over an irregular surface, to release compounds from the
water through oxidation, precipitation, or evaporation.
Agency – The U.S. Environmental Protection Agency.
Alkalinity – The capacity of water to neutralize acids, a property imparted by the water’s content
of carbonates, bicarbonates, hydroxides, and occasionally borates, silicates, and phosphates. It is
expressed in milligrams per liter of equivalent calcium carbonate.
Alum – A common name in water and wastewater treatment field for commercial-grade
aluminum sulfate (Al
2
(SO
4
)
3
· 14H
2
O).
Anion – The ion in an electrolyte solution that carries the negative charge and that migrates
toward the anode under the influence of a potential difference.
Anode – Positive pole of an electrolytic system, towards which anions (negatively charge ions)
migrate.
Aquifer – A natural underground layer, often composed of sand or gravel, that contains water.
B
Backwash – The process of reversing the flow of water back through the filter media to remove
the entrapped solids.
Basin – 1) A natural or artificially created space or structure, surface or underground, which has
a shape and character of confining material that enable it to hold water. The term is sometimes
used for a receptacle midway in size between a reservoir and a tank. 2) The surface area within a
given drainage system. 3) A shallow tank or depression through which liquids may be passed or
in which they are detained for treatment or storage.
14-1

Drinking Water Industry Report Section 14 – Glossary, Acronyms, and Abbreviations
Batch (intermittent) discharge – A discrete volume or mass of liquid or solid residuals that are
collected and discharged periodically. Equalization or slower discharge rate of batch discharges
may decrease negative impacts on the receiving stream.
Biochemical Oxygen Demand (BOD) – The quantity of oxygen used in the biochemical
oxidation of organic matter in a specified time, at a specified temperature, and under specified
conditions.
BOD
5
– Biochemical oxygen demand measured over a 5-day period.
Best professional judgment (BPJ) – The method used by permit writers to develop technology-
based NPDES permit conditions on a case-by-case basis using all reasonably available and
relevant data.
C
Cathode – Negative pole of an electrolytic system, towards which cations (positively charge
ions) migrate.
Cation – The ion in an electrolyte solution that carries the positive charge and that migrates
toward the cathode under the influence of a potential difference.
Centrate – Water separated from the solids by a centrifuge.
Centrifuge – A mechanical device in which centrifugal force is used to separate solids from
liquids and/or to separate liquids of different densities.
CFR – Code of Federal Regulations.
Clarification – Separation and concentration of solids from liquid/solid mixtures that are mostly
liquid (contrast with dewatering and thickening).
Clarifier – A large circular or rectangular tank or basin in which water is held for a period of
time, during which the heavier suspended solids settle to the bottom by gravity.
Clay – 1) Soil consisting of inorganic material, the grains of which have diameters smaller than
0.002 millimeters. 2) A mixture of earthy matter formed by the decay of certain minerals. The
composition of clays varies widely and dictates its use. It is sometimes used in water treatment to
aid coagulation and to remove tastes and odors.
Clean Water Act (CWA) – Federal legislation enacted by Congress to “restore and maintain the
chemical, physical, and biological integrity of the nation’s waters” (Federal Water Pollution
Control Act of 1972, as amended, 33 U.S.C. 1251 et seq).
Clear well – A reservoir for storage of finished water prior to distribution.
14-2

Drinking Water Industry Report Section 14 – Glossary, Acronyms, and Abbreviations
Coagulant – A chemical added to water that has suspended and colloidal solids to destabilize
particles, allowing subsequent floc formation and removal by sedimentation, filtration, or both.
Coagulation – As defined in 40 CFR 141.2, a process in which colloidal and suspended
materials are destabilized and agglomerated into flocs by using coagulant chemicals and mixing.
Colloids – Finely divided solids that will not settle but may be removed by coagulation of
biochemical action or membrane filtration; they are intermediate between true solutions and
suspensions.
Community Water System (CWS) – A water system that supplies drinking water to 25 or more
of the same people year-round.
Contaminant – Anything found in water (including microorganisms, minerals, chemicals,
radionuclides, etc.) that may be harmful to human health or the environment.
Continuous discharge – A volume or mass of liquid or solid residuals that are discharged at
constant flow without significant interruption.
Conventional filtration – As defined in 40 CFR 141.2, a series of processes including
coagulation, flocculation, sedimentation, and filtration resulting in substantial particulate
removal.
Conventional pollutants – Constituents of wastewater as determined by Section 304(a)(4) of the
CWA and EPA regulations. Conventional pollutants are classified as biochemical oxygen
demand, total suspended solids, oil and grease, fecal coliform, and pH.
D
Decant – To draw off the liquid from a basin or tank without stirring up the sediment in the
bottom.
Deep-well injection – Long-term or permanent disposal of untreated, partially treated, or treated
wastewaters by pumping the wastewater into underground formations of suitable character
through a bored, drilled, or driven well. Most commonly used for desalination plant concentrates.
Dewatering – Separation of liquid from liquid/solid mixtures that are predominantly solids,
often containing very low moisture content to start with (contrast with clarification and
thickening).
Dewatering processes – Mechanical and non-mechanical methods used to remove excess
liquids from residual solids in order to concentrate the solids. These methods include belt
presses, centrifuges, filter presses, vacuum presses, and lagoons.
Diatomaceous earth filtration –filtration method in which the filter media, diatomaceous earth,
is deposited on a support membrane or screen (called a septum) prior to each filter run (pre-coat).
The filter media is washed and wasted at the end of each filter run.
14-3

Drinking Water Industry Report Section 14 – Glossary, Acronyms, and Abbreviations
Direct discharge – The discernible, confined, and discrete conveyance of pollutants to United
States surface waters such as rivers, lakes, and oceans. See 40 CFR 122.2.
Direct discharger – A facility that discharges or may discharge treated or untreated wastewaters
into waters of the United States.
Direct filtration – As defined in 40 CFR 141.2, a series of processes including coagulation and
filtration, but excluding sedimentation, resulting in substantial particulate removal.
Direct recycle – The return of recycle flow within the treatment process without first passing
through treatment or equalization.
Discharge – The discernible, confined, and discrete conveyance of pollutants to: 1) United
States surface waters such as rivers, lakes, and oceans (“direct discharge”), or 2) a publicly
owned, privately owned, federally owned, combined, or other treatment works (“indirect
discharge”). Note that the definition at 40 CFR 122.2 excludes indirect discharges to publicly
owned treatment works; however, in this report, “discharge: refers to any direct or indirect
discharge.
Disinfectant – As defined in 40 CFR 141.2, any oxidant, including but not limited to chlorine,
chlorine dioxide, chloramines, and ozone added to water in any part of the treatment or
distribution process, that is intended to kill or inactivate pathogenic microorganisms.
Disinfection – As defined in 40 CFR 141.2, a process that inactivates pathogenic
microorganisms (such as bacteria, viruses, and protozoa) in water by chemical oxidants or
equivalent agents. Disinfection may be a chemical (commonly chlorine, chloramine, or ozone) or
physical process (e.g., ultraviolet light).
Disinfection by-products (DBPs) – Organic compounds formed by the reaction of the
disinfectant, natural organic matter, and the bromide ion. Regulated DBPs include
trihalomethanes, haloacetic acids, bromate, and chlorite.
Disposal – Intentional placement of residuals into or on any land, in either a permitted waste
disposal facility (e.g., landfill) or land application for agricultural or other purposes. Does not
include direct or indirect discharge of residuals.
Dissolved-air flotation – A method of solids separation, whereby a side stream is saturated with
air at high pressure and then injected into the flotation tank to mix with the incoming water
stream. As the air bubbles rise to the surface they attach to floc particles and create a sludge layer
at the surface of the tank, which is then removed for disposal.
Distribution system – A network of pipes leading from a treatment plant to customers'
plumbing systems.
14-4

Drinking Water Industry Report Section 14 – Glossary, Acronyms, and Abbreviations
E
Electrodialysis – A method of water treatment that utilizes electric current applied to permeable
membranes to remove minerals and salts from water.
Emergency discharge – A volume or mass of liquid or solid residuals that are discharged only
during extenuating circumstances (i.e., a treatment process malfunction). Also referred to as
upset or bypass discharge.
Equalization – A method used to control the flow of water or residuals stream by providing
storage and detention time between the point of origin and the return (or next) location of the
water or residuals stream. The water or residuals stream is then removed from the storage unit at
a controlled, uniform rate.
Evaporation – The process by which water or other liquid becomes a gas. Water from land
areas, bodies of water, and all other moist surfaces is absorbed into the atmosphere as a vapor.
Evaporation ponds – Dewatering and concentration of concentrates using evaporation.
F
Facility – All contiguous property and equipment owned, operated, leased, or under the control
of the same person or entity.
Filter press – A press operated mechanically for partially separating water from solid materials.
Filter-to-waste – Provision in a filtration process to allow the first filtered water, after
backwashing a filter, to be washed or reclaimed. Cleans filter prior to being put back into service
after backwashing.
Filtrate – The water separated from the solids by a filter press or the liquid that has passed
through a filter.
Filtration – As defined in 40 CFR 141.2, a process for removing particulate matter from water
by passage through porous media.
Finished water – As defined in 40 CFR 141.2, water that is introduced into the distribution
system of a public water system and is intended for distribution and consumption without further
treatment, except as treatment necessary to maintain water quality in the distribution system
(e.g., booster disinfection, addition of corrosion control chemicals).
Floc – Collections of smaller particles that have come together (agglomerated) into larger, more
settleable particles as a result of the coagulation-flocculation process.
14-5

Drinking Water Industry Report Section 14 – Glossary, Acronyms, and Abbreviations
Flocculation – As defined in 40 CFR 141.2, a process to enhance agglomeration or collection of
smaller floc particles into larger, more easily settleable particles through gentle stirring by
hydraulic or mechanical means.
Flow – 1) The movement of a stream of water or other mobile substance from place to place; a
stream of water; movement of silt, water, sand, or other material. 2) The fluid that is in motion.
3) The quantity or rate of movement of a fluid; discharge; total quantity carried by a stream. 4)
To issue forth or discharge.
Freeze-assisted sand beds – A structure used to freeze and thaw residuals to change the
characteristics to a more granular consistency that is easier to dewater. Most commonly used
with alum residuals.
G
Gravity filter – A rapid sand filter of the open type, the operating level of which is placed near
the hydraulic grade line of the influent and through which the water flows by gravity.
Ground water – Water in a saturated zone or stratum beneath the surface of land or water.
H
Haloacetic acids (HAA5) – The five haloacetic acid compounds include monochloroacetic acid,
dichloroacetic acid, trichloroacetic acid, monobromoacetic acid, and dibromoacetic acid. All are
disinfection by-products.
Hardness – A characteristic of water, imparted by salts of calcium, magnesium, and iron such as
bicarbonates, carbonates, sulfates, chlorides and nitrates, that cause curdling and increased
consumption of soap, deposition of scale in boilers, damage in some industrial processes, and
sometimes objectionable taste.
I
Impoundment – A pond, lake, tank, basin, or other space, either natural or man-made that is
used for storage, regulation, and control of water.
Indirect discharge – The discernible, confined, and discrete conveyance of pollutants to a
publicly owned treatment works. See 40 CFR 122.2.
Indirect discharger – A facility that discharges or may discharge wastewaters to a publicly
owned treatment works.
Influent water – Raw water plus any recycle streams.
14-6

Drinking Water Industry Report Section 14 – Glossary, Acronyms, and Abbreviations
Inorganic contaminants – Mineral-based compounds such as metals, nitrates, and asbestos.
These contaminants are naturally-occurring in some water, but can also arise through farming,
chemical manufacturing, and other human activities.
Ion – A charged atom, molecule, or radical, the migration of which affects the transport of
electricity through an electrolyte solution or, to a certain extent, through a gas. An atom or
molecule that has lost or gained one or more electrons. By such ionization it becomes electrically
charged. An example is the alpha particle.
Ion exchange (IX) – Process using a resin formulated to have capability to adsorb cationic or
anionic species, such as arsenate.
Ion-exchange regenerant – A chemical solution used to restore an exhausted bed of ion
exchange resins to the fully ionic (regenerated) form necessary for the desired ion exchange to
again take place effectively.
L
Lagoon – Basin or artificial impoundment containing solid or liquid material for purposes of
storage, treatment, or disposal.
Lime – Any of a family of chemicals consisting essentially of calcium hydroxide made from
limestone (calcite) that is composed almost wholly of calcium carbonate or a mixture of calcium
and magnesium carbonate.
Long-term average (LTA) – Average pollutant levels achieved over a period of time (EPA
recommends five years) by a plant or technology option.
M
Maximum Contaminant Level (MCL) – The highest level of a contaminant that EPA allows in
drinking water. MCLs ensure that drinking water does not pose either a short-term or long-term
health risk. EPA sets MCLs at levels that are economically and technologically feasible. States
can set MCLs that are more stringent than EPA MCLs.
Mechanical dewatering device – A device that operates mechanically to remove water from
residuals and produce a non-flowing residual. Examples include centrifuges, filter presses, belt
presses, plate press, and vacuum filters. Contrast with non-mechanical dewatering.
Median – In a statistical array, the value having as many cases larger in value as cases smaller in
value, or 50
th
percentile.
Membrane concentrate – The reject stream generated when the source water is passed through
a membrane for treatment.
MGD – Million gallons per day.
14-7

Drinking Water Industry Report Section 14 – Glossary, Acronyms, and Abbreviations
mg/L – Milligrams per liter.
Microfiltration – A method of water treatment that utilizes a membrane to separate micrometer
or submicrometer particles from a solution. The method clarifies water by trapping particles and
microorganisms in the membrane, while allowing dissolved substances to pass through with the
permeate (i.e., clean water).
Micron – A unit of length equal to one micrometer (μm). One millionth of a meter or one
thousandth of a millimeter. One micron equals 0.00004 of an inch.
Microorganisms – Tiny living organisms that can be seen only with the aid of a microscope.
Some microorganisms can cause acute health problems when consumed in drinking water. Also
known as microbes.
Monofill – An ultimate disposal technique for water treatment plant sludge in which the sludge
is applied to a landfill designed for sludge only.
N
North American Industry Classification System (NAICS) – NAICS was developed jointly by
the United States, Canada, and Mexico to provide comparability in statistics about business
activity across North America.
Nanofiltration – A method of water treatment that utilizes membranes and has the primary goal
of removing hardness, bacteria, viruses, and organic-related color.
Nonconventional pollutants – Pollutants that are neither conventional pollutants (40 CFR
401.16) nor priority pollutants (40 CFR 423 Appendix A).
Non-mechanical dewatering process – Process to separate solids from liquids in liquid/solid
mixtures without the use of mechanical devices, examples include sand or similar drying beds,
dewatering lagoons (lagoons designed for routine solids clearing), and freeze-assisted sand beds.
Contrast with mechanical dewatering and disposal, which includes long-term lagoons (i.e.,
lagoons that are cleaned of solids every 10 to 20 years or more).
Non-Transient, Non-Community Water System (NTNCWS) – A water system that supplies
water to 25 or more of the same people at least six months per year in places other than their
residences. Some examples are schools, factories, office buildings, and hospitals that have their
own water systems.
Nonwater quality environmental impact – Deleterious aspects of control and treatment
technologies applicable to point source category wastes, including, but not limited to air
pollution, noise, radiation, sludge and solid waste generation, and energy use.
14-8

Drinking Water Industry Report Section 14 – Glossary, Acronyms, and Abbreviations
National Pollutant Discharge Elimination System (NPDES) – As authorized by the Clean
Water Act, the NPDES permit program controls water pollution by regulating point sources that
discharge pollutants into waters of the United States. Point sources are discrete conveyances such
as pipes or man-made ditches. Individual homes that are connected to a municipal system, use a
septic system, or do not have a surface discharge do not need an NPDES permit; however,
industrial, municipal, and other facilities must obtain permits if their discharges go directly to
surface waters. In most cases, the NPDES permit program is administered by authorized states.
O
Off site – Outside the boundaries of a facility.
On site – The same or geographically contiguous property, which may be divided by a public or
private right-of-way, provided the entrance and exit between the properties is at a crossroads
intersection, and access is by crossing as opposed to going along the right-of-way.
Noncontiguous properties owned by the same company or locality but connected by a right-of-
way, which it controls, and to which the public does not have access, is also considered on-site
property.
Operating capacity – The maximum finished water production rate at a water treatment plant
approved by the state drinking water program authority.
Organic contaminants – Carbon-based chemicals such as solvents and pesticides that can get
into water through runoff from cropland or discharge from factories.
Outfall – The mouth of conduit drains and other conduits from which a facility discharges
effluent into receiving waters.
P
Pathogen – A disease-causing organism.
Permeability – The property of a material that permits appreciable movement of water through
it when it is saturated and the movement is actuated by hydrostatic pressure of the magnitude
normally encountered in natural subsurface water.
pH – An expression of the intensity of the basic or acid condition of a solution. Mathematically,
pH is the negative logarithm (base 10) of the hydrogen ion concentration, [H+]. [pH = log
(1/H+)]. The pH may range from 0 to 14, where 0 is most acidic, 14 most basic, and 7 neutral.
Natural waters usually have a pH between 6.5 and 8.5.
Pollutant – Under the Clean Water Act, a dredged spoil, solid waste, incinerator residue, filter
backwash, sewage, garbage, sewage sludge, munitions, chemical waste, biological materials,
certain radioactive materials, heat, wrecked or discarded equipment, rock, sand, cellar dirt, and
industrial, municipal, and agricultural waste discharged into water. This definition includes
14-9

Drinking Water Industry Report Section 14 – Glossary, Acronyms, and Abbreviations
residuals (including miscellaneous residuals) generated by water treatment plants. See 40 CFR
122.2.
Pollution prevention – The use of materials, processes, or practices that reduce or eliminate the
creation of pollutants or residuals.
Polymer – A synthetic organic compound with high molecular weight and composed of
repeating chemical units (monomers). Polymers may be polyelectrolytes (such as water soluble
flocculants), water-insoluble ion exchange resins, or insoluble uncharged materials (such as
those used for plastic or plastic-lined pipe).
Potable water – Water that does not contain objectionable pollution, contamination, minerals, or
infective agents and is considered satisfactory for domestic consumption.
Precipitative softening – A method of water treatment with the primary goal of reducing water
hardness. The method may include lime softening, sedimentation/precipitation, filtration, and
disinfection.
Presedimentation – Water treatment operation that is at the head of the plant. Its primary
purpose is to remove a significant percentage of suspended solids and other contaminants in the
water prior to other water treatment operations (e.g., conventional filtration, precipitative
softening). This water treatment operation may require a small addition of water treatment
chemicals, such as polymer coagulants (e.g., 0.5 to 1 mg/L), to aid sedimentation.
Priority pollutant – 126 compounds listed in 40 CFR Part 423 Appendix A that are a subset of
the toxic pollutants and classes of pollutants outlined pursuant to Section 307 of the CWA.
Process wastewater – Any water that, during source water treatment operations, comes into
direct contact with or results from the storage, production, or use of any raw material, by-
product, or waste product. Wastewater from equipment cleaning, direct-contact air pollution
control devices, rinse water, stormwater associated with industrial activity, and contaminated
cooling water are considered to be process wastewater. Process wastewater may also include
wastewater that is contract hauled for off-site disposal. Sanitary wastewater, uncontaminated
noncontact cooling water, stormwater not associated with industrial activity, and finished
drinking water are not considered to be process wastewater.
Public Water System (PWS) – Any water system that provides drinking water to at least 25
people for at least 60 days annually.
Publicly owned treatment works (POTW) – A treatment works as defined by Section 212 of
the CWA, which is owned by a state or municipality (as defined by Section 502(4) of the CWA).
This definition includes any devices and systems used in the storage, treatment, recycling and
reclamation of municipal sewage or industrial wastes of a liquid nature. It also includes sewers,
pipes and other conveyances, only if they convey wastewater to a POTW treatment plant. The
term also means the municipality, as defined in Section 502(4) of the CWA, that has jurisdiction
over the indirect discharges to and the discharges from such a treatment works.
Purchased water – Water obtained from a third-party vendor.
14-10

Drinking Water Industry Report Section 14 – Glossary, Acronyms, and Abbreviations
R
Radionuclides – Any man-made or natural element that emits radiation; may cause cancer after
many years of exposure through drinking water.
Raw water – Water in its natural state, prior to any treatment for drinking.
Recovery – The process of extracting some other useable constituent from one or more residuals
streams, for example, recovery of alum from coagulation sludge, lime from precipitative
softening sludge, and salt from concentrates.
Recycle – Process of returning liquid or combined liquid/solid residuals streams back to the
water treatment process (e.g., filter backwash recycling).
Regeneration – 1) In ion exchange, the process of restoring an ion exchange material to the state
employed for adsorption. 2) The periodic restoration of exchange capacity of ion exchange
media used in water treatment.
Reservoir – See “impoundment.”
Residuals – The solid, liquid, or mixed solid/liquid materials generated during source water
treatment. Examples of residuals include: sludges and wastewaters generated from
presedimentation, coagulation, flocculation, sedimentation, clarification, precipitative softening,
filter backwash operations, and filter-to waste; membrane reject wastewaters; ion exchange
resins and concentrate wastewaters; activated carbon wastes; and other miscellaneous residuals.
Residuals include those accumulated for batch discharge.
Residuals treatment – Any activity designed to change the character or composition of liquid
and solid residuals streams from water treatment processes as needed to render it amenable to
recycle, recovery, reduce its volume, or prepare it for transportation, storage, disposal, or
discharge. For example, this would include equalization, thickening, mechanical dewatering,
non-mechanical dewatering, and other processes defined separately.
Reverse osmosis – A method of water treatment that involves the application of pressure to a
concentrated solution that causes the passage of a liquid from the concentrated solution to a
weaker solution across a semipermeable membrane. The membrane allows the passage of the
solvent (water) but not the dissolved solids (solutes). This method is typically used, in
combination with pretreatment, for desalination and the removal of ions, radionuclides, bacteria,
and viruses.
14-11

Drinking Water Industry Report Section 14 – Glossary, Acronyms, and Abbreviations
S
Sand drying beds – Similar to evaporation ponds; evaporation is used to dewater and
concentrate liquid/solid residuals mixtures. One difference is that these structures are also
engineered to filter out solids so that a portion of the liquid is removed via subsurface infiltration
into ground water or the vadose zone.
Screen – A device with openings, generally of uniform size, used to retain or remove suspended
or floating solids in flowing water or wastewater and to prevent them from entering an intake or
passing a given point in a conduit. The screening element may consist of parallel bars, rods,
wires, grating wire mesh, or perforated plate. The openings may be of any shape, although they
are usually circular or rectangular.
Safe Drinking Water Information System (SDWIS) – Database containing information about
drinking water treatment systems and plants. There is a federal SDWIS and state SDWIS.
SDWIS identification numbers (or PWS IDs) are nine characters in length, with the first two
digits usually composed of the state abbreviation.
Sedimentation – Separation of solids and liquids from mixtures. Discrete and hindered settling
principally involves separation of solids from mixtures that are predominantly liquids, and these
processes are referred to as “clarification.” Sedimentation refers to the physical separation
process, in contrast to non-mechanical dewatering, which is a residuals treatment process, and
disposal, which is a residuals destination.
Sedimentation basin – A basin or tank in which water or wastewater containing settleable solids
is retained in order to remove by gravity a part of the suspended matter. Also called
sedimentation tank, settling basin, and settling tank.
Sequestering – To render inactive, such as chelation (binding of metal ion to form an inactive
metal compound).
Settleable solids – That matter in wastewater that will not stay in suspension during a
preselected settling period, such as one hour, but either settles to the bottom or floats to the top.
Settling – See “sedimentation.”
Settling basin – See “sedimentation basin.”
Site – See “facility.”
Slow sand filtration – As defined in 40 CFR 141.2, a process involving passage of raw water
through a bed of sand at low velocity (generally less than 0.4 meters/hour) resulting in
substantial particulate removal by physical and biological mechanisms.
Sludge – The accumulated solids separated from liquids during processing.
14-12

Drinking Water Industry Report Section 14 – Glossary, Acronyms, and Abbreviations
Sludge thickener – A tank or other piece of equipment designed to concentrate water treatment
sludges.
Source reduction – Any practice prior to recycling, treatment, or disposal that reduces the
amount of any hazardous substance, pollutant, or contaminant entering any residuals stream or
otherwise released into the environment. Source reduction can include equipment or technology
modifications, process or procedure modifications, substitution of raw materials, and
improvements in housekeeping, maintenance, training, or inventory control.
Source water – Intake (raw) water treated and/or distributed by utilities.
Spent filter backwash water – A stream containing particles that are dislodged from filter
media when water is forced back through a filter (backwashed) to clean the filter.
Standard Industrial Classification (SIC) – A numerical categorization system used by the U.S.
Department of Commerce to catalogue economic activity. SIC codes refer to the products, or
group of products, produced or distributed, or to services rendered by an operating establishment.
SIC codes are used to group establishments by the economic activities in which they are
engaged. SIC codes often denote a facility's primary, secondary, tertiary, etc. economic activities.
This system predated NAICS.
Supernatant – The water standing above a sediment or precipitate.
Surface waters – Waters of the United States, as defined at 40 CFR 122.2, including, but not
limited to, oceans and all interstate and intrastate lakes, rivers, streams, creeks, mudflats, sand
flats, wetlands, sloughs, prairie potholes, wet meadows, playa lakes, and natural ponds.
Suspended solids – Solid organic and inorganic particles that are held in suspension by the
action of flowing water and are not dissolved.
System – One or more water treatment facilities that produce and deliver finished water to
customers over the same distribution network.
T
Thickener supernatant – Thickener supernatant is the clarified water that exits the units after
particles have been allowed to settle out.
Thickening – Gravity separation and concentration of solids from liquid/solid mixtures that are
mostly solids.
Total suspended solids (TSS) – Solids in water that can be trapped by a filter. TSS can include
a wide variety of material, such as silt, decaying plant and animal matter, industrial wastes, and
sewage.
14-13

Drinking Water Industry Report Section 14 – Glossary, Acronyms, and Abbreviations
Total trihalomethanes (TTHM) – The trihalomethane compounds include trichloromethane
(chloroform), dibromochloromethane, bromodichloromethane and tribromomethane
(bromoform).
Toxic pollutants – those pollutants listed by the Administrator under CWA Section 307(a) and
listed at 40 CFR 401.15
Transient, Non-Community Water System (TNCWS) – A water system that provides water in
a places such as a gas station or campground where people do not remain for long periods of
time.
Treatment – Any method, technique, or process designed to change the physical, chemical, or
biological character or composition of any metal-bearing, oily, or organic waste in order to
neutralize such wastes, to render such wastes amenable to discharge, or to recover metal, oil, or
organic content from the wastes.
Trihalomethane (THM) – As defined in 40 CFR 141.2, one of the family of organic
compounds, named as derivatives of methane, wherein three of the four hydrogen atoms in
methane are each substituted by a halogen atom in the molecular structure.
Turbidity – The cloudy appearance of water caused by the presence of suspended and colloidal
matter that cause the scattering and adsorption of light. In the drinking water industry, a turbidity
measurement is used to indicate the clarity of water. Technically, turbidity is an optical property
of the water based on the amount of light reflected by suspended particles. WTPs may be able to
correlate turbidity to suspended solids. Because source water quality varies seasonally, weekly or
monthly correlations may be necessary.
U
Ultrafiltration – A method of water treatment that uses membranes in a pressure-driven process
for concentrating solutions containing colloids and higher molecular weight materials. The
method typically removes viruses, colloids, clays, bacteria, humic acids, and fulvic acids.
Underground injection – The technology of placing fluids underground, in porous formations
of rocks, through wells or other similar conveyance systems.
Utility – The public or private entity managing the business aspects of the production and
distribution of finished water from one or more water treatment systems (e.g., billing customers
for water service, paying utility employees and third-party vendors for services and products
provided to the utility, paying servicing fees for any outstanding debts). Customers are usually
more familiar with utility as a water supplier than a system, in those utilities that operate multiple
systems.
14-14

Drinking Water Industry Report Section 14 – Glossary, Acronyms, and Abbreviations
V
Vacuum-assisted drying beds – Dewatering technology in which a vacuum is applied to the
underside of porous media plates to remove the water from residuals.
Vadose zone – Area between the land surface and the water table.
W
Wastewater – See “process wastewater.”
Wastewater treatment – The processing of wastewater by physical, chemical, biological, or
other means to remove specific pollutants from the wastewater stream, or to alter the physical or
chemical state of specific pollutants in the wastewater stream. Treatment is performed for direct
or indirect discharge of treated wastewater, recycle of treated wastewater to the same process
that generated the wastewater, or for reuse of the treated wastewater in another process.
Water treatment – Any activity associated with altering the character or composition of source
water prior to storage, transmission, distribution, and consumption by public water utility
consumers. This treatment takes place at a water treatment plant (see definition).
Water treatment plant (WTP) – A water treatment facility in which ground water, surface
water, or other source water is processed to produce potable water for storage, transmission,
distribution, or consumption by public water utility consumers. For the purposes of the industry
review, this term does not encompass off-facility treatment stations (e.g., booster chlorination
stations, fluoridation stations, corrosion control treatment stations) or off-site water transfer
infrastructure (e.g., tunnel transferring turbid water from one watershed body to another
waterbody upstream of the facility, water towers that are downstream of the facility).
Water treatment system – One or more water treatment plants that produce and deliver finished
water to customers over the same distribution network.
Water treatment utility – See “utility”.
Watershed – The land area from which water drains into a stream, river, or reservoir.
Well – A bored, drilled or driven shaft whose depth is greater than the largest surface dimension;
a dug hole whose depth is greater than the largest surface dimension; an improved sinkhole; or
a subsurface fluid distribution system.
Z
Zero discharge – Disposal of process residuals other than by direct discharge to a surface water
or by indirect discharge to a publicly owned, privately owned, federally owned, combined, or
other treatment works.
14-15

Drinking Water Industry Report Appendix A
APPENDIX A
SURVEY DESIGN AND CALCULATION OF NATIONAL ESTIMATES

Drinking Water Industry Report Appendix A
APPENDIX A
SURVEY DESIGN AND CALCULATION OF NATIONAL
ESTIMATES
One of the data collection activities undertaken by EPA was a survey of drinking
water treatment facilities, known as the Water Treatment Plant Questionnaire. This appendix
provides detailed information about the statistical methods used in conducting the survey.
Section A.1 provides a discussion of the sample frame created for the survey. Section A.2
presents the statistical sample design used to select treatment systems for inclusion in the survey.
Section A.3 describes the response rates for Part A of the questionnaire (technical questions).
Section A.4 presents the statistical methods used to calculate national estimates of various
operating characteristics based upon the responses to Part A. The national estimates are provided
in Section 3 of this report. Section A.5 provides references.
A.1 SAMPLE FRAME
This section provides an overview of the sample frame used to select systems for
the survey. Further information about the sample frame and survey design can be found in EPA’s
Information Collection Request Supporting Documentation (U.S. EPA, 2006).
For the survey, EPA originally considered approximately 160,000 public water
systems that collectively provide 90 percent of the nation’s drinking water. After examining
existing data sources, EPA reduced the target population for the survey to a relatively small
subset to reduce industry’s burden and to obtain information from systems most likely to produce
residuals. Specifically, the target population included all public water systems except those in the
following groups:
• Systems serving fewer than 10,000 people were excluded because, while
they account for 93 percent of community water systems (CWSs), EPA
estimates they contribute less than nine percent of the residuals from the
industry (U.S. EPA, 2006).
A-1

Drinking Water Industry Report Appendix A
• CWSs that do not produce residuals were excluded either because they do
not treat the source water, or they treat it in a manner that is unlikely to
produce residuals.
• About 10 non-community water systems (NCWS) serving populations
greater than 10,000 were excluded because they do not serve permanent
resident populations and, thus, may have different discharge practices and
financial characteristics.
The sample frame from which the sample was drawn was derived from EPA’s
Safe Drinking Water Information System (SDWIS), a database that stores routine data about the
nation’s drinking water. The data in SDWIS were combined with additional data from other
sources, such as the 2000 CWSS (Community Water System Survey) – which has operational
and financial data not available in SDWIS – and the May 14, 1996 Information Collection Rule
to support future regulation of microbial contaminants, disinfectants, and disinfection byproducts
– which has engineering and operations information not available in SDWIS. The database
created from these sources provided a complete listing of all systems in the United States. The
sample frame database also contained information about each treatment plant within a system.
Information maintained in the sample frame database at the system level included:
• Public Water System identification number PWSID, name, owner, and
contact information;
• Primary water source;
• Population served; and
• Community served.
Information contained in the sample frame database at the treatment facility (plant) level
included:
• Facility Registration System identification number FRSID, name, and
location;
• Associated PWSID;
• Primary water source;
A-2

Drinking Water Industry Report Appendix A
• Estimated population served;
• Treatment method information; and
• Discharge information.
The data associated with systems was considered to be of high quality. The quality of the
information about individual plants, however, varied, with high-quality information about
primary water source and treatment method but poor quality information about discharge and
population served.
The plants that met the criteria presented above formed the target population.
However, data collected by the CWSS, as well as other data available in SDWIS, focused on
information at the system level rather than information for each individual plant. Thus, EPA
decided to create a sample frame of water treatment systems and to request information from
each system about its member plants. Specifically, the questionnaire that was sent to each
sampled system asked a series of qualifying questions to determine if the system had any plants
within the target population. Systems were only required to complete the remaining questions if
they had plants within the target population.
EPA determined that the sample frame of treatment systems has a nearly 100
percent coverage rate for the target population. The sample frame database provided a complete
listing of all community water systems in the United States serving at least 10,000 people at the
time it was finalized. Because no treatment plant in a system serving fewer than 10,000 people
could serve more than 10,000 people, the sample frame should contain all systems that have
members of the target population. There is, however, a small probability that between the times
that the sample frame database was finalized and the sample was selected, the size of the
population served by a single-facility system could have moved from fewer than 10,000 to over
10,000. In such a case, the facility would be in the target population but its system would not be
in the sample frame. EPA has judged the likelihood of such a case occurring as being very small.
A-3

Drinking Water Industry Report Appendix A
A.2 SAMPLE DESIGN
This section describes the sample design for the survey. A sample design
identifies the way in which the survey data are to be collected. EPA chose to use a stratified
sample design that required only some systems to respond. The systems and plants identified
from the stratified sample are statistically representative of all systems and plants in the target
population.
A.2.1 Statistical Design and Strata
EPA used a stratified sample design to select treatment systems to receive a
questionnaire. Stratification is performed by selecting one or more characteristics of interest and
dividing the members of the population into “strata” that are defined by those characteristics.
Generally, the sample frame identifies these characteristics or provides a basis to reasonably
assign characteristics to each population member. Stratified sampling consists of selecting a
probability-based sample from within each stratum, then combining them to constitute the total
sample. There are several benefits that can result from a stratified sampling approach, including:
• Ensuring that the sample contains representatives from every stratum;
• Improving the precision of parameter estimates (if the strata are defined
appropriately);
• Allowing important parameters to be estimated at the stratum level; and
• Allowing certain subpopulations of particular interest to be sampled at a
greater rate than others.
To select systems to receive questionnaires, EPA used the following stratification
variables, which were available from the sample frame:
• Size of population served;
• Primary water source (surface water or ground water); and
• Treatment type.
A-4

Drinking Water Industry Report Appendix A
Size of population served was selected for use in stratification because it served as a surrogate
for the volume of water treated and the volume of residuals that are produced. EPA considered
two population size groups. The chosen cut-point was 50,000, because a review of existing
literature showed that this cut-point is commonly used in evaluating drinking water systems.
Primary water source was selected as a stratification variable because substantial differences can
exist in the amount and type of residuals that a plant produces between treating surface water and
treating ground water. Surface water, which is taken from above-ground sources such as rivers,
lakes, wetlands, or estuaries, is more vulnerable to contamination and usually needs treatment
before it is safe to drink. Ground water, which is pumped from underground aquifers through
drilled wells or from springs, is protected by layers of soils and other subsurface materials and
often needs only minimal treatment.
EPA considered larger systems (i.e., those serving populations greater than
50,000) to be more likely to have residual management (or treatment) than small systems. Thus,
for larger systems, EPA considered treatment type as an additional stratification variable,
because it can affect the volume and characteristics of the residuals generated. Treatment type
often depends on the plant’s size, its source water quality, and other environmental factors, such
as climate. It may also depend on the experience of the plant operator or engineer with the
treatment technologies. In addition, state or federal regulatory requirements may affect
technology choices. EPA considered the following four treatment types:
• Softening or ion exchange (SOFT/IX);
• Conventional and direct filtration, including coagulation/flocculation
(CONV);
• Membrane technology, including reverse osmosis, ultrafiltration, and
electrodialysis (MEM); and
• Other, including filtration without coagulation/flocculation, activated
carbon, activated alumina, and aeration (OTHER).
In addition to these stratification variables, EPA also considered whether a
system’s water quality region would be an appropriate stratification variable, because geographic
differences in source water characteristics could affect treatment and residuals generation.
A-5

Drinking Water Industry Report Appendix A
However, EPA determined that use of water quality region in conjunction with the other
stratification variables would have resulted in an extremely inefficient statistical sample design
with large variance estimates. Instead, EPA incorporated water quality region into the sample
selection mechanism in a manner that would not disproportionally affect variance. First, EPA
sorted the plants by water quality region within each stratum defined by the four stratification
variables. EPA then drew a systematic sample from each cell, which involves selecting every k
th
plant, where k is determined randomly according to the selection rate. In this manner, EPA
ensured that the sample was reasonably diverse from a geographical perspective while achieving
an efficient sample design.
A.2.2 Target Precision Expected from the Sample Design
For the final sample design, EPA selected a total of 616 systems to receive the
questionnaire. The number of systems selected was based on requirements concerning the
precision of the survey estimates. The precision depends on both the sample design and the
sample size. For the drinking water treatment (DWT) industry survey, the precision requirement
was defined in terms of the width of a 95 percent confidence interval for an estimated proportion.
Because a proportion of 0.5 (or 50 percent) results in the largest possible variance for the
binomial distribution, EPA used that case in defining the target precision. Based upon EPA’s
simulation, the sample would be expected, with 95 percent confidence, to yield sufficient data to
estimate the value of an unknown proportion to within ±0.05 of its true value for the target
population. This precision target will hold when the proportion’s true (unknown) value is equal
to 0.5, and even greater precision is expected when the true value of the proportion is not equal
to 0.5. Furthermore, the simulation estimated that a statistical sample of 593 systems distributed
among the sampling strata would result in a sample of 673 plants from the target population,
which would then represent an estimated 2,402 plants in the total population. (After including
additional systems into its sample frame, EPA slightly increased the sample size to 616 systems.)
A.2.3 Sample Selection Procedure
The statistical selection of systems to receive questionnaires, as noted above, was
done systematically within each stratum after sorting by water quality region to ensure
A-6

Drinking Water Industry Report Appendix A
geographic diversity within the sample. In addition, EPA sampled at a higher rate among strata
that were mostly likely to produce residuals. EPA sampled more large systems than small ones
and more surface water systems than ground water systems. In summary, the sample design had
the following characteristics:
• Systems for large populations (greater than 50,000) were four times more
likely to be selected than systems serving small populations (between
10,000 and 50,000);
• Systems with surface water as the primary water source were three times
more likely to be selected than systems with ground water;
• Larger populations were selected on the basis of (i.e., stratified by)
primary water source and treatment method, while smaller populations
were stratified by primary water source only; and
• A minimum of five systems were selected from each cell.
The specific strata, the number of systems within each stratum, the sampling
fraction, and number of systems that were selected in each stratum for the DWT industry survey
as part of the statistical sample are shown in Table A-1. In the original sample design, EPA
included an allotment for a judgment sample of 25 systems that would not have been part of the
statistical estimates. However, EPA later chose to increase the statistical sample size and used
the allotment from the judgment sample for this purpose.
A-7

Drinking Water Industry Report Appendix A
Table A-1. DWT Survey Strata, Population Size, and Sample Size
Stratum
Sample Information
Size of
Population
Served
Primary
Water
Source
Treatment Method
Sampling
Fraction
(Percent)
a
Number of
Systems in
Frame
Number of
Systems in
Statistical Sample
10,001-50,000
Ground
Any
6
625
37
Surface
Any
18
1,044
187
Total
Sub-total
1,669
224
More than 50,000
Ground
Conventional
Filtration
100 8 8
Membrane
100
5
5
Other
24
82
20
Softening
24
50
12
Ion Exchange
100
5
5
Sub-total
150
50
Surface
Conventional
Filtration
72 288 208
Membrane
72
26
19
Other
72
86
62
Softening
72
67
49
Ion Exchange
100
4
4
Sub-total
471
342
Sub-total
621
392
Total
2,290
616
a – A minimum of five systems were sampled from each stratum.
A.3 RESPONSE STATUS
This section describes the response rates, non-response evaluations, bias
considerations, and unusual situations requiring adjustments to the Part A responses.
A.3.1 Response Rates
Table A-2 shows the final disposition of Part A responses to the survey by
stratum. In the rest of the document, references to the “survey” and “responses” pertain only to
the Part A (technical) responses. Table A-2 addresses the number of non-responding systems but
does not address non-responses to individual questions. Data were analyzed without any
adjustments made for missing responses to individual questions.
A-8

Drinking Water Industry Report Appendix A
Despite EPA efforts to obtain the missing information through repeated phone
calls and email communications with the survey respondents, 46 surveys had one or more
missing responses and/or responses needing clarification at the completion of the survey. An
additional 28 surveys had missing information; however, these surveys included 27 surveys from
Puerto Rico and one survey from New York. The next section describes EPA’s evaluation of
potential patterns in the non-responses.
A-9

Drinking Water Industry Report Appendix A
Table A-2. DWT Survey Part A: Strata, Population Size, and Sample Size, and Response Information for Systems
Stratum
Sample Information
Response/In-Scope Information For SYSTEMS
Size of
Population
Served
Primary
Water
Source
Treatment
Method
Sampling
Fraction
(Percent)
a
Number
of
Systems
in Frame
Number of
Systems in
Sample
Number of
Responses
Received
Number of
Non-
Respondents
Number
In-Scope
Respondents
Estimated
Population
In-Scope
10,001-
50,000
Ground
Any
6
625
37
17
20
17
283
Surface
Any
18
1,044
187
125
62
125
694
Sub-total
1,669
244
142
82
142
978
More than
50,000
Ground
Conventional
Filtration
100 8 8 5 3 5 5
Membrane
100
5
5
3
2
3
3
Other
24
82
20
8
12
8
33
Softening
24
50
12
11
1
11
46
Ion Exchange
100
5
5
3
2
3
3
Sub-total
150
50
30
20
30
90
Surface
Conventional
Filtration
72 288 208 175 33 174 242
Membrane
72
26
19
14
5
14
19
Other
72
86
62
43
19
42
58
Softening
72
67
49
37
12
37
51
Ion Exchange
100
4
4
2
2
2
2
Sub-total
471
342
271
71
269
373
Sub-total
621
392
301
91
299
463
Total
2,290
616
443
173
441
1,441
a – A minimum of five systems were sampled from each stratum.
A-10

Drinking Water Industry Report Appendix A
A.3.2 Non-Response Evaluation
This section describes EPA’s evaluation of potential non-response patterns to the survey.
Such patterns are evaluated as a function of known information about all systems that were
included in the sample. Some information about the sampled systems is available in the sampling
frame. Specifically, the sample frame includes (1) information that was used to define sampling
strata, and (2) other system characteristics that were not used to create sampling strata. For the
DWT survey, EPA used the size of the population served, the primary water source, and the
treatment method to create the sampling strata. Other information available in the DWT survey
sampling frame used to examine potential non-response patterns includes:
• EPA Region,
• Water Quality Region,
• State, and
• Type of owner.
EPA combined the information from the sampling frame with the results of the
DWT survey to prepare summaries of non-response rates compared with system characteristics.
Tables A-3 through A-7 provide summaries of non-response rate of the sampled systems in
relation to several of the variables. In particular:
• Table A-3 shows the non-response information by stratum,
• Table A-4 shows the non-response information by EPA Region,
• Table A-5 shows the non-response information by Water Quality Region,
• Table A-6 shows the non-response information by state, and
• Table A-7 shows the non-response information by type of owner.
Each of the five tables contains the following information:
• Variable level,
• Total number of systems in the DWT survey sample,
• Number and percent of sampled systems whose responses indicated that
they were unqualified for participation in the survey,
• Number of qualified systems responding to the survey (and percent of all
sampled systems not known to be unqualified),
A-11

Drinking Water Industry Report Appendix A
• Number of non-responding systems (which includes those systems that did
not return the questionnaire and those systems that returned the
questionnaire but only provided partial responses (response to Question 1,
indicated they were qualified to participate in the survey), and
• Number of known in-scope non-respondents (i.e., only responded to
Question 1 and indicated they were in-scope).
As noted in the bullets above, there were two types of non-responding systems
defined for the summaries: those for which no responses were received, and those that sent
partial responses. The latter group comprises the “known in-scope non-respondents” systems.
The percentages shown in Tables A-3 through A-7 are calculated differently for various
columns. For the number of unqualified systems, the denominator of the percentage is the
number of systems sampled. For the number of responding and non-responding systems that
were not known to be unqualified, the denominator of the percentage subtracted the number of
unqualified systems from the total number sampled, or equivalently, the sum of the numbers of
systems that were known to be qualified and the systems for which there was no response at all.
For example, in the first row of Table A-3, the percentage of unqualified systems is equal to 19
divided by 37, and the percentage of non-responding systems is equal to 3 divided by 18 (37 –
19).
Upon examining Tables A-3 through A-7, EPA noted the following situations
with increased rates of non-response:
• In Table A-3, most strata have between 20 and 40 percent non-response
rates. The two exceptions, at 50 and 75 percent, are the two ion exchange
strata for populations greater than 50,000. Both of these strata have no
more than five members.
• In Table A-4, EPA Region 2 has a significantly higher non-response rate
(62%) than the other regions, due in part to non-response by systems in
Puerto Rico.
• In Table A-5, systems with a Water Quality Region specification of “e”
have a 95% non-response rate, while all other regions have non-response
rates of less than 40%. The “None” category includes Puerto Rico and
Guam.
• In Table A-6, the states that have a greater than 50% non-response rate
include Maryland and Puerto Rico. Mississippi, Nebraska, and Utah have
50% response rates, but there were only 2 qualified systems sampled in
each.
• In Table A-7, non-response rates were similar for all types of owner.
A-12

Drinking Water Industry Report Appendix A
In examining all five tables together, it is clear that the primary place where there
is a significant non-response pattern is caused by the partial responses from the systems in Puerto
Rico.
A.3.3 Bias Considerations
Non-response bias occurs when the survey responses that would have been received from
a group of sampled subjects who do not respond to the survey are systematically different than
the actual responses received from the subjects who did complete the survey. Thus, non-response
bias can be present any time there are non-respondents, regardless of whether there are any
obvious patterns in respondents and non-respondents. However, in the case of the DWT survey,
there is a clear pattern of non-response from the Puerto Rico systems that could have an
associated non-response bias due to potential differences in characteristics between Puerto Rico
systems and the responding systems. The presence of non-response bias related to partial
responses from Puerto Rico systems may lead to inaccurate national estimates of variables
measured by the survey. This is due to the fact that the different values of survey responses from
Puerto Rico are not incorporated into the national estimate, causing under- or over-estimates of
the variables of interest. As explained below, EPA considers that inaccurate national estimates
are unlikely, even considering the non-responses from Puerto Rico.
There are three available options for addressing the issue of potential response
bias due to the partial responses from the Puerto Rico systems that were included in the sample.
Each of the options, with information about assumptions and consequences are presented below.
(1) Assume that Puerto Rico systems are similar to others within strata.
The purpose of placing systems into strata for the sample selection (and into domains for
the analysis) is to create sets of systems that should have similar survey responses based
on the fact that they have similar characteristics with regard to population size, primary
water source, and treatment method. Because EPA placed the Puerto Rico systems into
strata based on these characteristics, EPA would assume that all other systems in the
A-13

Drinking Water Industry Report Appendix A
strata would produce similar results to those that would be expected from the Puerto Rico
systems. If it can be assumed that the strata are homogenous, EPA would expect no non-
response bias because other similar systems are present. As a result, the methods that
EPA have used to adjust for unit non-response will be adequate to account for the Puerto
Rico systems, and no additional adjustments to the survey analysis will be required.
(2) Make additional efforts to obtain data from some of the Puerto Rico systems.
The electronic/mailed survey used with the DWT survey failed to obtain all the necessary
data from the Puerto Rico systems to include in the survey response database. This may
be due to one of two likely reasons: (a) the systems were unwilling to respond to a
particular question in the questionnaire, or (2) the systems could not reliably respond to a
questionnaire in English. To counter these reasons, EPA could choose one of two
alternatives to collect data from the Puerto Rico systems. The first alternative is for EPA
to follow up with several of the systems and attempt to collect some data. EPA did
contact the system, but did not receive a response.. A second alternative is for EPA to
translate the questionnaire into Spanish and resubmit it to the sampled Puerto Rico
systems. If the Puerto Rico systems did not respond, EPA would then need to address
non-response associated with these systems. If some responses were received from
follow-up efforts, EPA can adjust the analyses to incorporate the new data, including a
revision of the non-response adjustments to the survey weights.
(3) Re-define the target population to represent the 50 states and DC.
Because the most significant non-response issue is that of partial responses from Puerto
Rico, which is a territory rather than a state, EPA could choose to change the scope of the
survey results to apply to only the 50 United States and the District of Columbia. This
would also result in the elimination of any data from systems from other territories, such
as Guam and Saipan, as well as Puerto Rico. If this approach was taken, EPA would need
to revise the survey weights to account for changes in the probability of selection and
redo the analysis incorporating the new survey weights.
A-14

Drinking Water Industry Report Appendix A
EPA considers Option 1 to be the most reasonable alternative for three reasons. First, the sample
design was developed to place Puerto Rico systems within strata based on characteristics they
shared with other systems. Thus, EPA expected Puerto Rico responses to be similar to those for
other systems within the associated strata, and thus, it would not be appropriate to redefine the
target population (i.e., Option 3). Second, a logistic regression analysis of the non-response rates
in Table 1 shows that there are no statistically significant differences in the non-response rates
among the various strata. Thus, the strata that contain the Puerto Rico systems (small surface-
water systems, large surface-water systems with conventional treatment, and large surface-water
systems with “other” treatment) are similar to the other strata with regard to non-response rates.
Third, during permit support activities, EPA visited and evaluated several Puerto Rico systems.
The engineering team noted many similarities to systems operated elsewhere. While these
observations are subjective, they support a finding that Option 1 is a reasonable assumption.
A-15

Drinking Water Industry Report Appendix A
Table A-3. Survey Part A: Non-Response by Stratum
Stratum
Number in
Sample
Number
Unqualified
(%)
Number
Respondents
(%)
Number Non-
Respondents
(%)
Number Non-
Respondents in
Scope
Size of
Population
Served
Primary
Water
Source
Treatment Method
10,001-50,000
Ground Any 37 19 (51) 15 (83) 3 (17) 1
Surface Any 187 21 (11) 118 (71) 48 (29) 26
Sub-total 224 40 (18) 133 (72) 51 (28) 27
More than
50,000
Ground
Conventional
Filtration
8 1 (13) 6 (86) 1 (14) 1
Membrane 5 0 (0) 3 (60) 2 (40) 0
Other 20 10 (50) 8 (80) 2 (20) 1
Softening 12 0 (0) 8 (67) 4 (33) 3
Ion Exchange 5 1 (20) 1 (25) 3 (75) 3
Sub-total 50 12 (24) 26 (68) 12 (32) 8
Surface
Conventional
Filtration
208 5 (2) 147 (72) 56 (28) 37
Membrane 19 1 (5) 11 (61) 7 (39) 3
Other 62 13 (21) 27 (55) 22 (45) 18
Softening 49 1 (2) 32 (67) 16 (33) 9
Ion Exchange 4 0 (0) 2 (50) 2 (50) 0
Sub-total 342 20 (6) 219 (68) 103 (32) 67
Sub-total 392 32 (8) 245 (68) 115 (32) 75
Total
616 72 (12) 378 (69) 166 (31) 102
A-16

Drinking Water Industry Report Appendix A
Table A-4. Survey Part A: Non-Response by EPA Region
EPA
Region
Number
in Sample
Number Unqualified
(%)
Number
Respondents
(%)
Number Non-
Respondents
(%)
Number Non-
Respondents in
Scope
01 38 5 (13) 28 (85) 5 (15) 2
02 62 10 (16) 20 (38) 32 (62) 24
03 65 4 (6) 37 (61) 24 (39) 14
04 147 16 (11) 93 (71) 38 (29) 25
05 82 3 (4) 68 (86) 11 (14) 7
06 68 10 (15) 38 (66) 20 (34) 12
07 27 1 (4) 19 (73) 7 (27) 2
08 26 3 (12) 17 (74) 6 (26) 2
09 82 16 (20) 45 (68) 21 (32) 13
10 19 4 (21) 13 (87) 2 (13) 1
Table A-5. Survey Part A: Non-Response by Water Quality Region
Water Quality
Region
Number
in
Sample
Number
Unqualified
(%)
Number
Respondents
(%)
Number Non-
Respondents
(%)
Number Non-
Respondents in
Scope
Appalachia 33 5 (15) 24 (86) 4 (14) 2
Central North 24 2 (8) 18 (82) 4 (18) 2
Central South 31 2 (6) 21 (72) 8 (28) 2
Florida 31 9 (29) 13 (59) 9 (41) 3
Great Lakes 74 3 (4) 60 (85) 11 (15) 7
Mid Atlantic 72 3 (4) 47 (68) 22 (32) 17
North East 116 18 (16) 67 (68) 31 (32) 15
North Mountain 4 0 (0) 4 (100) 0 (0) 0
North West 18 4 (22) 12 (86) 2 (14) 1
South East 50 3 (6) 32 (68) 15 (32) 10
South Mountain 26 2 (8) 18 (75) 6 (25) 2
South West 74 15 (20) 38 (64) 21 (36) 13
Texas 41 4 (10) 23 (62) 14 (38) 9
None 22 2 (9) 1 (5) 19 (95) 19
A-17

Drinking Water Industry Report
Table A-6. Survey Part A: Non-Response by State/Territory
State
Number in
Sample
Number
Unqualified
(%)
Number
Respondents
(%)
Number Non-
Respondents
(%)
Number Non-
Respondents in
Scope
AL
15
1 (7)
12 (86)
2 (14)
2
AR
9
3 (33)
5 (83)
1 (17)
1
AZ
8
0 (0)
7 (88)
1 (13)
0
CA
68
14 (21)
36 (67)
18 (33)
11
CO
15
0 (0)
10 (67)
5 (33)
2
CT
8
0 (0)
7 (88)
1 (13)
1
FL
31
9 (29)
13 (59)
9 (41)
3
GA
21
0 (0)
13 (62)
8 (38)
4
GU
1
1 (100)
0
0 (NA)
0
HI
1
0 (0)
1 (100)
0 (0)
0
IA
9
0 (0)
6 (67)
3 (33)
1
ID
1
0 (0)
1 (100)
0 (0)
0
IL
20
1 (5)
16 (84)
3 (16)
2
IN
11
2 (18)
7 (78)
2 (22)
1
KS
7
1 (14)
4 (67)
2 (33)
0
KY
16
1 (6)
10 (67)
5 (33)
5
LA
4
0 (0)
3 (75)
1 (25)
0
MA
20
5 (25)
12 (80)
3 (20)
0
MD
7
0 (0)
3 (43)
4 (57)
1
ME
1
0 (0)
1 (100)
0 (0)
0
MI
8
0 (0)
8 (100)
0 (0)
0
MN
8
0 (0)
8 (100)
0 (0)
0
MO
9
0 (0)
8 (89)
1 (11)
0
MP
1
1 (100)
0
0 (NA)
0
MS
3
1 (33)
1 (50)
1 (50)
0
MT
1
0 (0)
1 (100)
0 (0)
0
NC
29
1 (3)
21 (75)
7 (25)
6
ND
2
0 (0)
2 (100)
0 (0)
0
NE
2
0 (0)
1 (50)
1 (50)
1
NH
4
0 (0)
4 (100)
0 (0)
0
NJ
19
6 (32)
7 (54)
6 (46)
2
NM
3
2 (67)
1 (100)
0 (0)
0
NV
3
0 (0)
1 (33)
2 (0)
2
NY
24
4 (17)
13 (65)
7 (35)
3
OH
25
0 (0)
20 (80)
5 (20)
3
OK
11
1 (9)
6 (60)
4 (40)
2
OR
11
3 (27)
6 (75)
2 (25)
1
PA
35
3 (9)
19 (59)
13 (41)
8
PR
19
0 (0)
0 (0)
19 (100)
19
RI
3
0 (0)
2 (67)
1 (33)
1
SC
11
1 (9)
6 (60)
4 (40)
4
SD
3
2 (67)
1 (100)
0 (0)
0
TN
21
2 (10)
17 (89)
2 (11)
1
TX
41
4 (10)
23 (62)
14 (38)
9
UT
3
1 (33)
1 (50)
1 (50)
0
VA
20
1 (5)
13 (68)
6 (32)
5
VT
2
0 (0)
2 (100)
0 (0)
0
WA
7
1 (14)
6 (100)
0 (0)
0
WI
10
0 (0)
9 (90)
1 (10)
1
A-18

Drinking Water Industry Report Appendix A
State
Number in
Sample
Number
Unqualified
(%)
Number
Respondents
(%)
Number Non-
Respondents
(%)
Number Non-
Respondents in
Scope
WV
3
0 (0)
2 (67)
1 (33)
0
WY
2
0 (0)
2 (100)
0 (0)
0
Table A-7. Survey Part A: Non-Response by Type of Owner
Owner Type
Number in
Sample
Number
Unqualified
(%)
Number
Respondents
(%)
Number Non-
Respondents
(%)
Number Non-
Respondents in
Scope
Federal
Government
8 2 (25) 4 (67) 2 (33) 2
Local
Government
503 56 (11) 307 (69) 140 (31) 85
Private 92 10 (11) 61 (74) 21 (26) 13
Public/Private 4 1 (25) 2 (67) 1 (33) 0
State
Government
5 2 (40) 2 (67) 1 (33) 1
Unknown 4 1 (25) 2 (67) 1 (33) 1
A-19

Drinking Water Industry Report Appendix A
A.3.4 Assumptions Used to Modify Responses
While analyzing the survey responses, it was necessary for EPA to make
assumptions about the responses under certain circumstances. These circumstances, and EPA’s
actions, were:
• If a system had multiple similar plants but a common residuals treatment
system, EPA treated the multiple plants as a single plant having one
residuals treatment system.
• If one plant discharged to another plant within the same system for
residuals treatment but the finished water processes were significantly
different (e.g., desalination and conventional), the process was recorded as
multiple plant information. For the site without a residuals treatment
system, the discharge was a zero or indirect discharge to the other site. For
the site with the residuals treatment system, the discharges from both
plants were influent to the residuals treatment system.
Additionally, some treatment facilities provided unusual responses. These systems, their
issues, and EPA’s resolution are noted below.
• The cities of Phoenix and Mesa, AZ, co-owned the Val Vista plant
(AZ0407025). As a result, separate sets of economic responses were
submitted for each system, while only one set of technical responses was
submitted for the plant. In this situation, two “pseudo-plants” were
created, one within each system, and the technical responses were
apportioned to each of the pseudo-plants proportionally to the percentage
of operating costs paid by each system.
• The Hillsboro and Joint Water Commission (JWC) plant is jointly owned
by the cities of Hillsboro (PWS OR4100379), Tigard (OR4100878),
Beaverton (OR4100081) Tualatin (OR4100665), and Forest Grove
(OR4100305). Only Hillsboro was included in the sample, but they
provided data for the jointly-owned plant. The plant information was
scaled down to represent only the City of Hillsboro and not the other four
cities that own and use water from the plant.
• The sample included both the City of Poughkeepsie (NY1330291) and
Town of Poughkeepsie (NY1302774). The data for the Town of
Poughkeepsie was received first, so it was included in the analysis. The
City of Poughkeepsie data was a duplicate of the Town of Poughkeepsie
A-20

Drinking Water Industry Report Appendix A
data. Thus, the City of Poughkeepsie was determined to be out-of-scope
and was not included in the analysis.
• The Lancaster County (SC) Water system (SC292001) and the Union
County (NC) Water System (NC0190413) share ownership of a single
plant. Only the SC system was included in the sample. It uses 40 percent
of the total water production from the plant, while the NC system uses the
other 60 percent. The survey response includes complete technical
information for the plant but economic data for only the SC system. The
technical data was scaled down to represent only the SC system.
A.4 STATISTICAL METHODS FOR CALCULATING ESTIMATES
The following subsections discuss the methods that were used to calculate the
national estimates of the technical and economic characteristics of DWT plants and systems.
Section A.4.1 discusses the survey weights that were calculated for the DWT survey. Section
A.4.2 discusses the methods used to organize the results for presentation in this report. Finally,
Section A.4.3 presents the methods for calculating the national estimates. A complete discussion
of the statistical methods can be found in Cochran (1977).
A.4.1 Survey Weights
Survey weights are applied during the analysis of survey data to obtain unbiased
estimates of the population parameters of interest. Because a sample of DWT systems was
selected, the results for any given respondent may represent more than one plant or system. The
weight indicates the number of plants or systems that are represented by the respondent. These
weights are used in calculating unbiased estimates of the national estimates. The survey weights
have been obtained in the manner prescribed by Office of Management and Budget (2006).
The subsections that follow describe the calculation of the survey weights for the
DWT survey. Section A.4.1.1 presents the method used for calculating the base survey weights.
Section A.4.1.2 presents the methods used for adjusting the weights for ineligible and non-
responding systems. Section A.4.1.3 provides a table showing the actual weights that were
calculated for the DWT survey.
A-21

Drinking Water Industry Report Appendix A
A.4.1.1 Base Survey Weight Calculation
The first step in obtaining the survey weights required to ensure unbiased
estimates of population parameters was to calculate base survey weights. These base survey
weights are defined to be the inverse of the probability of selection. That is, for stratum h,
h
h
h
n
N
w =
, (Eq. A.1)
where N
h
is the number of systems in the stratum and n
h
is the number of systems.
A.4.1.2 Eligibility and Non-Response Adjustments to Survey Weights
Because not all systems responded to the survey, and also because some of the
systems included in the sample were not eligible to participate, the base survey weights may
inaccurately represent the systems within each stratum. To ensure that the weights are
representative, the base weights are adjusted to account for ineligible systems that are in the
sample and population and to account for systems that did not respond to the survey. Potential
respondents can be divided into four categories:
1. Eligible respondents (r);
2. Eligible non-respondents (e);
3. Ineligible respondents (i); and
4. Systems with unknown eligibility (u).
For the DWT survey, it was not possible to determine whether non-respondents were eligible or
not eligible, so all non-respondents were placed into the category of unknown eligibility (i.e., e =
0).
The eligibility and non-response adjustments were made in two steps. In the first
step, the base weight was adjusted for ineligibility. The specific equation for obtaining the
eligibility-adjusted survey weights was
hh
hhh
hh
ur
iur
ww
+
++
⋅=
, (Eq. A.2)
A-22

Drinking Water Industry Report Appendix A
where r
h
is the number of eligible respondents, u
h
is the number of systems with unknown
eligibility, and i
h
is the number of ineligible respondents. In the second step, the eligibility-
adjusted weight was adjusted for non-response using the following equation
h
hh
hh
r
ur
ww
+
⋅=
. (Eq. A.3)
In this case, the value of u
h
represents all non-respondents.
A.4.1.3 Final Survey Weights
Table A-8 contains the base and adjusted survey weights that were used in the
analysis of the DWT survey data. These weights were calculated using Equations (A.1), (A.2),
and (A.3).
Table A-8. Survey Part A: Calculated Survey Weights
Size of
Population
Served
Primary
Water
Source
Treatment
Method
Base
Survey
Weights
Eligibility
Adjustment
Non-
Response
Adjustment
Final
Survey
Weights
10,001-50,000
Ground Any 16.89 2.31 1.07 41.67
Surface Any 5.58 1.30 1.22 8.85
More than
50,000
Ground
Conventional
Filtration
1.00 1.14 1.17 1.33
Membrane 1.00 1.67 1.00 1.67
Other 4.10 2.22 1.13 10.25
Softening 4.17 1.09 1.38 6.25
Ion Exchange 1.00 1.25 4.00 5.00
Surface
Conventional
Filtration
1.38 1.13 1.25 1.96
Membrane 1.37 1.36 1.27 2.36
Other 1.39 1.38 1.67 3.19
Softening 1.37 1.20 1.28 2.09
Ion Exchange 1.00 2.00 1.00 2.00
A.4.2 Organization of Results using Analysis Domains
The sample design for the DWT survey defined the way in which the survey
participants were selected and data were to be collected. For this survey, DWT systems were
A-23

Drinking Water Industry Report Appendix A
selected using a stratified sampling design, with the size of the population served, primary water
source, and primary treatment method used as stratifying variables. Technical data were
collected for qualifying treatment plants that were part of the selected systems, and economic
data were collected for the systems themselves.
There were many cases observed where the characteristics of a particular plant
differed from that of the system as a whole. For example, there were some systems that served
over 50,000 people that had individual plants serving fewer than 50,000 people. Similarly, a
system that used primarily surface water could have had a plant that used primarily ground
water. Because EPA’s interest concerning the technical operational data is at the plant level, EPA
chose to present the results of the technical data based on the characteristics of the plants rather
than based on the survey strata (which was based on system-level characteristics). EPA defined a
set of “domains” of a plant for presenting the national estimates of the technical data. These
domains correspond to the sampling strata; that is, the domains are based on the number of
people served, the primary water source, and the treatment method used at the plant.
For population served, the plant domain that was used in technical analyses
presented in Section 3.2 was defined using the population served by the plant. Specifically,
plants were divided into one of two groups: those that served between 10,000 and 50,000, and
those that served more than 50,000. For the system-level economic analyses presented in Section
3.3, system domains for population served were defined by summing the population counts
served by each individual plant within the system (for which data were available). Systems were
placed into one of two categories: those that served between 10,000 and 50,000 and those that
served more than 50,000.
For primary water source, the domain for each plant used in the technical analyses
of Section 3.2 was defined as the water source with the largest percentage as reported in
Question 2e of the survey. The system domain used for the economic analyses of Section 3.3 was
defined to be the domain for the largest plant in the system, as defined by gallons of water
produced from Question 2d of the questionnaire. There were several instances where this method
for defining system domain may have produced inaccurate results. For example, consider a
system that has three plants, one that produces 3 million gallons per day (MGD) using 100%
A-24

Drinking Water Industry Report Appendix A
surface water, and two others that each produce 2 MGD using 100% ground water. The system
as a whole uses more ground water, but it is classified into the surface-water domain based on
the use of the primary source from the largest plant. There are other similar scenarios that could
result in misclassification of the primary water source. Despite the potential misclassifications,
EPA chose to define system domains for primary water source using the characteristics of the
plant with the largest water production.
For treatment method, plant domains were defined using the treatment methods
provided in their response to Question 2f of the survey. If there was a single treatment method
listed (or there were several different methods that fell under the same grouping as shown in
Section A.2.1) the plant was assigned to that treatment method grouping. There were several
cases where a plant indicated that it used more than one treatment method (in different
groupings). Table A-9 shows the types of multiple-treatment-methods plants that responded to
the survey and the number of plants in each group. Based on an examination of the individual
cases, each of these plants was assigned by an expert to one of the treatment methods. Table A-9
also shows the way the plants were assigned to treatment methods.
Table A-7. Survey Part A: Assignment of Multiple-Treatment Plants to Treatment Types
Treatment Types
Treatment Type for
Analyses
Number of Cases
Conventional Filtration plus some other method of treatment
Conventional
451
Dechlorination, Primary Disinfection, and Ultrafiltration
Membrane
1
Other, Primary Disinfection, and Reverse osmosis
Membrane
1
Nanofiltration and Primary Disinfection
Membrane
3
Primary Disinfection and Reverse osmosis
Membrane
1
Precipitative and Primary Disinfection
Softening
4
Ion exchange and Primary Disinfection
Softening
2
Dechlorination, Microfiltration, Presedimentation, and
Primary Disinfection
Other 1
Microfiltration, Presedimentation, and Primary Disinfection
Other
1
Dechlorination, Other, and Primary Disinfection
Other
2
Other, Presedimentation, and Disinfection
Other
1
Microfiltration and Primary Disinfection
Other
2
Other and Primary Disinfection
Other
11
A-25

Drinking Water Industry Report Appendix A
A.4.3 National Estimates Based Upon Part A Responses
National estimates were calculated directly from Part A of the survey results for
each of the questions, except Question 2j and the questions requesting contact information. EPA
presents the methods used to calculate the national estimates in the report. Section A.4.3.3
contains a discussion of the methods used to obtain baseline estimates for pollutant loadings.
A.4.3.1 Estimates and Standard Errors
Several types of population estimates were calculated from the DWT survey data.
For numeric data (e.g., flow volume, number of connections), these estimates included minima,
maxima, medians, means, and totals. The category of numeric variables also included several
cases where two or more numeric variables were combined. For example, cost per connection
was a numeric variable that was calculated by dividing the total cost for a plant by the total
number of connections for the plant. For “characteristic” data (i.e., categorical responses to
questions asking whether plants or systems had certain characteristics), the types of estimates
calculated included proportions/percentages and counts. Although the DWT survey was designed
as a stratified sample, stratified sampling estimators were not directly relevant because the results
are reported for domains rather than for the strata.
The formulas used to calculate the estimates are provided in the subsections
below. Several terms are common to these formulas, including:
• H is the total number of strata;
• n
h
is the number of sampled plants or systems in stratum h;
• f
h
is the sampling rate for stratum h;
• y
hi
= the measurement of interest collected from the i
th
sampled member
of stratum h;
• w
hi
= the survey weight associated with the i
th
sampled member of stratum
h, which is equal to
h
w
from Equation (A.3);
A-26

Drinking Water Industry Report Appendix A
•
otherwise
DDomain
tobelongs
i
hify
z
hi
hi
),
(
=
0
; and
•
otherwise
D
Domaintobelongsihifw
v
hi
hi
),(
=
0
.
All of the formulas discussed below are implemented in SAS
®
using the procedures
UNIVARIATE for minima, maxima, and median, and SURVEYMEANS for means, totals,
counts, and proportions (SAS Institute Inc., 2008).
Minima and Maxima
The population minimum value of a continuous variable was estimated using the
smallest observed value of the variable among all strata. Similarly, the population maximum was
estimated using the largest observed value of the variable among all strata. Minimum and
maximum values within strata were estimated using the smallest and largest observed value
within the stratum.
Medians
The population median, or 50
th
percentile, was estimated using the following
formula:
<⋅<
⋅=+⋅
=
∑∑∑
∑ ∑
+
===
+
= =
+
1
111
1
1 1
1
50
50
5050
i
j
j
n
j
j
i
j
ji
i
j
n
j
jjii
wwwify
wwifyy
y
.
.)(.
ˆ
)(
)()(
.
, (Eq. A.4)
where y
(i)
indicates the i
th
smallest value within the domain or stratum and w
i
is the weight
associated with that value.
Means
The formula that was used to calculate estimates of population means for domains
can be written as
A-27

Drinking Water Industry Report Appendix A
∑∑
∑∑
= =
= =
⋅
=
H
h
n
i
hi
H
h
n
i
hihi
D
h
h
v
zv
y
1 1
1 1
. (Eq. A.5)
The variance of this estimated mean is calculated as
( )
∑ ∑
= =
−⋅
−
−⋅
=
H
h
n
i
hhi
h
hh
D
h
rr
n
fn
yV
1 1
2
1
1 )(
)(
, (Eq. A.6)
where
( )
∑
∑
= =
−⋅
=
H
h
n
i
hi
Dhihi
ij
h
v
yzv
r
1
1
(Eq. A.7)
and
h
n
i
hi
h
n
r
r
h
∑
=
=
1
. (Eq. A.8)
The standard error of the estimated mean is the square root of the variance shown in Equation
(A.6).
Totals
The formula for estimates of population totals, Y
D
, for domains can be written as
∑∑
= =
⋅=
H
h
n
i
hihiD
h
zvY
1 1
. (Eq. A.9)
The variance of this estimated total is
( )
∑ ∑
= =
−⋅⋅
−
−
⋅
=
H
h
n
i
hhihi
h
h
h
D
h
az
v
n
fn
YV
1
1
2
1
1 )
(
)(
, (Eq. A.10)
where
h
n
i
hihi
h
n
zv
a
h
∑
=
⋅
=
1
. (Eq. A.11)
The standard error of the estimated total is the square root of the variance.
A-28

Drinking Water Industry Report Appendix A
Ratios
There were several cases where new variables were defined as ratios of two
measured variables. For example, in Table 3-35, the estimated sales revenue per volume was
defined using the total sales and the total water volume. For these types of estimates, EPA
defined a new variable as the ratio of the two component variables, calculated this ratio for each
responding plant or system, and used Equations (A.5) through (A.8) to calculate the estimates of
the mean ratio and its standard error.
Plant Counts
Estimates for the number of plants or systems within domains are obtained using
the equations presented for domain totals. In this case, the values of the continuous variable for
which totals are calculated are replaced with indicator variables corresponding to whether the
plant or system possesses the characteristic of interest. For example, if we define y
hi
= 1 if the i
th
plant in stratum h uses conventional filtration and 0 if it does not for all sampled plants, equation
(A.9) can be used to estimate the total number of plants within each domain that use
conventional filtration. Equation (A.10) can also be used to calculate the variability of the plant
counts as well as its standard error.
Proportions/Percentages
Estimates of population proportions for domains are calculated in a similar
manner to plant counts using Equations (A.5) through (A.8) applied to the indicator variables
defined for plant counts. The overall national estimates of proportions (using the strata rather
than domains) are calculated as
∑
=
=
12
1h
hh
st
pwp
ˆˆ
, (Eq. A.12)
where
h
h
h
n
a
p =
ˆ
. (Eq. A.13)
The variance of the estimated population proportion is
A-29

Drinking Water Industry Report Appendix A
( )
( )
h
h
h
hh
hst
f
n
pp
wpV −
−
−
=
∑
=
1
1
1
12
1
2
ˆˆ
)
ˆ
(
(Eq. A.14)
and its standard error is the square root of the variance.
A.4.3.2 Confidence Intervals
In many cases, there will be interest in obtaining confidence intervals for the
national parameters rather than “point” estimates of the parameters. Confidence intervals provide
a range of probable values that the population parameter could be. The following formula is used
to calculate a confidence interval for a domain mean:
)(
2/ DD
ySEzyCI ⋅+=
α
, (Eq. A.15)
where z
α
/2
is the upper 100(
α
/2) percentile of a standard normal distribution and
)(
D
ySE
is the
standard error for
D
y
. For other population parameters, confidence intervals are obtained using
the associated estimates and their standard errors in Equation (A.15).
A.4.3.3 Baseline Pollutant Loading Estimates
In addition to providing basic estimates using the specific questions on the DWT
survey, EPA examined the source of pollutant loadings. For this analysis, plants were divided
into domains based on five parameters: treatment plant type, separation of residuals employed,
discharge status, population served (as a surrogate for flow volume), and use of chlorination.
Treatment method was defined as in Section A.2.1. For the other parameters, EPA classified
plants in the following manner:
• Separation of residuals was “Yes” if the plant used thickening, drying,
mechanical dewatering, non-mechanical dewatering, evaporation ponds,
equalization, or sediment tank ponds to treat residuals.
• Discharge status was direct, indirect, or both, based on the plant’s
response to Question 2k of the questionnaire.
A-30

Drinking Water Industry Report Appendix A
• Four size categories were defined based on the population they served:
10,000 to 50,000, 50,001 to 100,000, 100,001 to 500,000, and more than
500,000.
• Chlorination plants included those that used some form of calcium
hypochlorite, chloramination, free chlorine, gaseous chlorine, or sodium
hypochlorite as their primary disinfection.
EPA prepared separate tables for chlorination and non-chlorination plants.
A.5 REFERENCES
Cochran, W.G., 1977. Sampling Techniques. Wiley & Sons, New York.
Office of Management and Budget, 2006. Guidance on Agency Survey and Statistical
Information Collections. Office of Information and Regulatory Affair.
SAS Institute, Inc., 2008. SAS/STAT 9.2 User's Guide. SAS Institute, Inc., Cary, North Carolina.
U.S. Environmental Protection Agency (EPA), 2006. Supporting Statement: Survey of Drinking
Water Treatment Facilities. Office of Water.
A-31

Drinking Water Industry Report Appendix B
APPENDIX B
COMPOSITION OF COMMON DRINKING WATER TREATMENT CHEMICALS ILLUSTRATING
PRODUCTION IMPURITIES
(Source: American Water Works Association (AWWA), David A. Cornwell, Michael J.
Macphee and Richard Brown, 2002. Trace Contaminants in Drinking Water Chemicals, AWWA
Research Foundation)

Drinking Water Industry Report Appendix B
Table B-1. Composition of Aluminum-Based Coagulants Illustrating Production
Impurities
Pollutant
Median Concentration (mg/kg dry weight)
a
Standard Alum
Low-iron Alum
Polyaluminum Chloride
(PACl)
Aluminum
90,000
89,400
153,911
Antimony
<0.8
<0.8
<1.2
Arsenic
<2.06
<2.00
<2.6
Barium
<0.10
<0.10
0.21
Cadmium
<0.1
<0.1
<0.2
Calcium
62
62
149
Chromium
66
0.6
0.6
Cobalt
<0.20
<0.15
<0.41
Copper
1.86
0.21
1.34
Iron
1,300
39
91
Lead
<4.1
<4.1
<4.1
Magnesium
33
14
41
Manganese
2.5
0.8
3.2
Mercury
<0.82
1.03
1.44
Molybdenum
<1.7
<1.7
<1.4
Nickel
0.90
0.41
1.65
Phosphorus
89
<4
<9
Potassium
7.5
7.7
10.7
Selenium
<4.1
<5.1
<2.1
Silicon
52
14
56
Silver
<0.82
<0.82
<1.65
Sodium
247
577
546
Strontium
1.03
0.41
0.41
Sulfur
Not analyzed
Not analyzed
Not analyzed
Tin
<2.1
<2.1
<2.7
Titanium
27
1.2
3.0
Vanadium
39
0.20
6
Yttrium
<0.41
<0.30
<0.52
Zinc
3
16
14
Zirconium
12
0.4
0.9
Source: American Water Works Association (AWWA), David A. Cornwell, Michael J. Macphee and Richard
Brown, 2002. Trace Contaminants in Drinking Water Chemicals, AWWA Research Foundation.
a – The less than sign denotes that the value was below sample-specific method detection limits (MDL); the value
listed is the MDL.
B-1

Drinking Water Industry Report Appendix B
Table B-2. Composition of Iron-Based Coagulants Illustrating Production Impurities
Pollutant
Single Sample Concentration (mg/kg dry weight)
a
Ferric Chloride
Ferric Sulfate
SPL#1
SPL#2
TiO2#1
Aluminum
1,289
19,737
3,158
82
Antimony
9
6
7
<4
Arsenic
<5
<3
<3
<4
Barium
0.3
1
18
1
Cadmium
1.0
1.0
1.0
1.4
Calcium
158
974
153
371
Chromium
124
111
100
<1
Cobalt
17
8
22
8
Copper
95
82
6
<0.4
Iron
355,263
305,263
315,789
228,866
Lead
53
<5
<13
41
Magnesium
55
316
316
173
Manganese
1,868
1,079
2,553
169
Mercury
<5
<3
5
No data
Molybdenum
<1
3
18
<0.8
Nickel
58
39
11
23
Phosphorus
29
263
42
163
Potassium
26
23
50
56
Selenium
No data
<3
<3
No data
Silicon
12
<1
15
8
Silver
<5
<2
<2
<4
Sodium
211
395
895
47
Specific Gravity
1.4
1.4
1.4
No data
Strontium
2
4
9
2
Sulfur
158
2,579
63
206,186
Tin
<5
<3
14
<4
Titanium
2
24
10,789
13
Vanadium
95
79
1,553
227
Yttrium
<1
<0.5
<0.5
<0.8
Zinc
45
53
258
37
Zirconium
10
8
4,474
6
Source: American Water Works Association (AWWA), David A. Cornwell, Michael J. Macphee and Richard
Brown, 2002. Trace Contaminants in Drinking Water Chemicals, AWWA Research Foundation.
a – The less than sign denotes that the value was below sample-specific method detection limits (MDL); the value
listed is the MDL.
SPL – Steel pickle liquor derived.
TiO2 – Derived during manufacture of titanium oxide.
B-2

Drinking Water Industry Report Appendix B
Table B-3. Composition of Potassium Permanganate in Samples from One Study
Illustrating Production Impurities
Pollutant
Single Sample Concentration (mg/kg dry weight)
a
Product #1
Product #2
Aluminum
560
610
Antimony
<10
<10
Arsenic
<10
<10
Barium
11
100
Cadmium
<1
<1
Calcium
39
230
Chromium
44
72
Cobalt
<2
<2
Copper
<1
<1
Iron
320
520
Lead
<49
<400
Magnesium
<0.3
<0.3
Manganese
333,000
336,000
Mercury
79
<10
Molybdenum
24
12
Nickel
26
31
Potassium
238,000
234,000
Selenium
73
80
Silicon
750
1,000
Silver
82
79
Sodium
370
3,300
Strontium
1
7
Tin
<10
<10
Titanium
4
9
Vanadium
<2
<2
Yttrium
2
<2
Zinc
2
3
Zirconium
3
<2
Source: American Water Works Association (AWWA), David A. Cornwell, Michael J. Macphee and Richard
Brown, 2002. Trace Contaminants in Drinking Water Chemicals, AWWA Research Foundation.
a – The less than sign denotes that the value was below sample-specific method detection limits (MDL); the value
listed is the MDL.
B-3

Drinking Water Industry Report Appendix B
Table B-4. Composition Data for Organic Polymers Illustrating Production Impurities
Pollutant
Concentrations for 12 Organic Polymers (mg/kg wet weight)
a
Minimum
Maximum
Median
Aluminum
<0.50
2,200
<40
Antimony
<1
<240
<76
Arsenic
<1
<240
<76
Barium
<0.01
<3
<1
Cadmium
<0.10
<25
<8
Calcium
0.50
120
73
Chromium
<0.20
<49
<16
Cobalt
<0.20
<49
<16
Copper
<0.10
<25
<8
Iron
<0.20
<340
<17
Lead
<1
<460
<78
Magnesium
<0.30
54
7
Manganese
<0.02
8
3
Mercury
<1
<240
<76
Molybdenum
<0.20
<49
<16
Nickel
<0.04
<49
<16
pH (standard units)
4.2
6.8
5.7
Potassium
<4.00
<970
<324
Selenium
<1
<240
<160
Silicon
<1
130
<52
Silver
<0.80
<190
<61
Sodium
85
27,000
940
Specific Gravity (no units)
0.99
1.14
1.04
Strontium
<0.02
<3
<1
Sulfur
13
4,100
695
Tin
<1
<240
<76
Titanium
<0.10
490
<8
Total organic carbon (TOC)
4,178 (one sample)
No data
No data
Vanadium
<0.20
<49
<16
Yttrium
<0.20
<49
<16
Zinc
<0.10
230
<12
Zirconium
<0.20
140
<17
Source: American Water Works Association (AWWA), David A. Cornwell, Michael J. Macphee and Richard
Brown, 2002. Trace Contaminants in Drinking Water Chemicals, AWWA Research Foundation.
a – The less than sign denotes that the value was below sample-specific method detection limits (MDL); the value
listed is the MDL.
B-4

Drinking Water Industry Report Appendix B
Table B-5. Composition Data for Lime Products Illustrating Production Impurities
Pollutant
Single Sample Concentration (mg/kg as Ca(OH)2 dry weight)a
Hydrated lime #1
Hydrated lime #2
Hydrated lime #3
Pebble lime
Aluminum
2,154
2,700
2,267
1,135
Antimony
<15
<2
<67
<2
Arsenic
<15
<2
<67
<2
Barium
77
13
27
5
Cadmium
<1.5
0.2
<6.7
<0.2
Calcium
507,692
495,000
493,733
495,676
Chromium
<3.1
2
<13.3
1
Cobalt
<3.1
0.4
<13.3
<0.3
Copper
1.5
2
<6.7
0.5
Iron
846
1,600
1,067
560
Lead
<15
<4
<333
<3
Magnesium
7,231
7,700
16,667
4,465
Manganese
35
23
73
16
Mercury
<15
<2
<6.7
<2
Molybdenum
4.6
<0.4
<13.3
<0.3
Nickel
<3.1
1
<13.3
0.5
Potassium
785
860
1,067
832
Selenium
<15
<2
<67
<2
Silicon
4,154
4,600
6,467
1,665
Silver
<12
<2
<53
<2
Sodium
1,277
49
3,000
22
Strontium
338
240
307
212
Tin
<15
<2
<67
<2
Titanium
74
66
87
26
Vanadium
3.1
3
<13.3
2
Yttrium
<3.1
1
<13.3
1
Zinc
9
4
6.7
2
Zirconium
3.1
3
<13.3
2
Source: American Water Works Association (AWWA), David A. Cornwell, Michael J. Macphee and Richard
Brown, 2002. Trace Contaminants in Drinking Water Chemicals, AWWA Research Foundation.
a –The less than sign denotes that the value was below sample-specific method detection limits (MDL); the value
listed is the MDL.
B-5

Drinking Water Industry Report Appendix B
Table B-6. Composition Data for Caustic Soda Illustrating Production Impurities
Pollutant
Single Sample Concentration (mg/kg dry weight)
a
50%
50%
25%
50%
Aluminum
8
5
<2
<1
Antimony
<2
<2.0
<4
<2
Arsenic
<2
<2.0
<4
<2
Barium
<0.02
<0.02
0.2
0.6
Cadmium
<0.2
<0.2
<0.4
<0.2
Calcium
<1
1
24
6
Chromium
<0.4
<0.4
<0.8
1
Cobalt
<0.4
<0.4
<0.8
<0.4
Copper
0.2
<0.2
<0.4
<0.2
Iron
1
2.0
6
36
Lead
<2
<2.0
<20
<20
Magnesium
0.2
0.4
4
2
Manganese
<0.04
<0.04
<0.08
0.6
Mercury
<2
<2.0
<4
<2
Molybdenum
<0.4
<0.4
<0.8
0.6
Nickel
<0.4
<0.4
<0.8
1
pH (standard units)
9.94
11.10
12.80
10.70
Phosphorus
112
<8
<4
7
Potassium
320
1,180
560
980
Selenium
<2.0
<2.0
<4
<2
Silicon
340
480
44
166
Silver
<2
<1.6
<3
<2
Sodium
508,000
510,000
444,000
508,000
Specific gravity (no units)
1.53
1.53
1.22
1.52
Strontium
0.4
0.2
4
2.4
Sulfur
18
36
60
168
Tin
<2
<2
<4
<2
Titanium
<0.2
<0.4
0.8
<0.2
Vanadium
<0.4
<0.4
<0.8
<0.4
Yttrium
<0.4
<0.4
<0.8
<0.4
Zinc
0.4
<0.2
1.2
0.4
Zirconium
<0.4
<0.4
<0.8
<0.4
Source: American Water Works Association (AWWA), David A. Cornwell, Michael J. Macphee and Richard
Brown, 2002. Trace Contaminants in Drinking Water Chemicals, AWWA Research Foundation.
a – The less than sign denotes that the value was below sample-specific method detection limits (MDL); the value
listed is the MDL.
B-6

Drinking Water Industry Report Appendix C
APPENDIX C
POTW PERCENT REMOVALS
(Sources: U.S. Environmental Protection Agency (U.S. EPA), 1982. Fate of Priority Pollutants
in Publicly Owned Treatment Works (EPA 440/1-82/303, September 1982 and U.S. EPA, 1994.
National Risk Management Research Laboratory (NRMRL) Treatability Database Version 5.0,
Cincinnati, OH)

Drinking Water Industry Report Appendix C
Table C-1. POTW Removals
DWT Parameter Name
POTW Removal (fraction)
Aluminum, Dissolved
0.91
Aluminum, Total
0.91
Aluminum, Unknown
0.91
Ammonia, Total
0.39
Ammonia, Unionized
0.39
Ammonia, Unknown
0.39
Arsenic
0.6577
Barium, Unknown
0.5515
Benzene
0.95
Cadmium, Total
0.9005
Chlorine, Free
1
Chlorine, Total Residual
1
Chloroform
0.73
Chromium
0.8033
Copper, Dissolved
0.842
Copper, Total
0.842
Copper, Unknown
0.842
Dichloroboromomethane
0.6424
Lead, Total
0.7745
Lead, Unknown
0.7745
Manganese, Total
0.406
Manganese, Unknown
0.406
Manganese, Dissolved
0.406
Mercury, Unknown
0.9016
Nickel, Unknown
0.5144
Zinc, Total
0.7914
Selenium
0.3433
Zinc, Unknown
0.7914
Aluminum
0.91
Ammonia
0.39
Barium
0.5515
Cadmium
0.9005
Copper
0.842
Lead
0.7745
Manganese
0.406
Mercury
0.9016
Nickel
0.5144
Zinc
0.7914
Phosphorus, Total
0.69
Phosphorus as P
0.69
C-1

Drinking Water Industry Report Appendix C
Table C-1. POTW Removals
DWT Parameter Name
POTW Removal (fraction)
Trihalomethane
0.73
Trihalomethane, Total
0.73
Trihalomethane, Unknown
0.73
Mercury, Total
0.9016
Boron
0.3042
Fluoride
0.6135
Iron
0.8199
Oil & Grease
0.8608
Chlorodibromomethane
0.0073
Magnesium
0.1414
Nitrogen, Total
0.5741
TKN
0.5741
Hydrogen Sulfide
0.5741
TSS
0.8955
Turbidity
0.8955
BOD
0.8912
Calcium
0.0854
Chlorides
0.5741
Nitrates
0.5741
Nitrites
0.5741
Phosphates
0.3252
Settleable Solids
0.8955
SS
0.8955
CBOD5
0.8912
Sulfate
0.8461
Total Organic Carbon
0.7028
TDS
0.08
Bromoform
0.73
Haloacetic Acids
0.73
DWT – Drinking Water Treatment.
C-2

Drinking Water Industry Report Appendix D
APPENDIX D
TOXIC WEIGHTING FACTORS (TWFS)
(Source: U.S. EPA. 2006. Toxic Weighting Factor Development in Support of CWA 304(m)
Planning Process. Washington, DC. (June). EPA-HQ-OW-2004-0032-1634)

Drinking Water Industry Report Appendix D
Table D-1. Toxic Weighting Factors
DWT Parameter Name
TWF (toxic weighted pounds per pound of
pollutant)
Aluminum, Dissolved
0.064691216
Aluminum, Total
0.064691216
Aluminum, Unknown
0.064691216
Ammonia, Total
0.00111
Ammonia, Unionized
0.00111
Ammonia, Unknown
0.00111
Arsenic
4.041333333
Barium, Unknown
0.001990757
Benzene
0.031678038
Boron, Total
0.17721519
Cadmium, Total
23.1168
Calcium, Unknown
0.000028
Chlorides
2.43478E-05
Chlorine, Free
0.509162182
Chlorine, Total Residual
0.509162182
Chlorine, Unknown
0.509162182
Chlorodibromomethane
0.044483378
Chloroform
0.002078389
Chromium
0.075696709
Copper, Dissolved
0.634822222
Copper, Total
0.634822222
Copper, Unknown
0.634822222
Dichloroboromomethane
0.032918058
Fluoride, Total
0.035
Fluoride, Unknown
0.035
Hydrogen Sulfide
2.801446667
Iron, Dissolved
0.0056
Iron, Total
0.0056
Iron, Unknown
0.0056
Lead, Total
2.24
Lead, Unknown
2.24
Magnesium
0.000865533
Manganese, Dissolved
0.07043299
Manganese, Total
0.07043299
Manganese, Unknown
0.07043299
Mercury, Total
117.1180233
Mercury, Unknown
117.1180233
Nickel, Unknown
0.108914308
Nitrogen, Total
Phosphate, Total
D-1

Drinking Water Industry Report Appendix D
Table D-1. Toxic Weighting Factors
DWT Parameter Name
TWF (toxic weighted pounds per pound of
pollutant)
Sulfate
0.0000056
Zinc, Total
0.046886
Nitrates
0.000746667
Nitrites
0.0032
BOD
Oil and Grease
Phosphorus, Total
Radium, Combined
Salinity
Selenium
1.121344
Settleable Solids
TDS
Total Organic Carbon
Trihalomethane, Total
TSS
Zinc, Unknown
0.046886
Aluminum
0.064691216
Ammonia
0.00111
Barium
0.001990757
Cadmium
23.1168
Calcium
0.000028
Copper
0.634822222
Fluoride
0.035
Iron
0.0056
Lead
2.24
Manganese
0.07043299
Mercury
117.1180233
Nickel
0.108914308
Zinc
0.046886
DWT – Drinking Water Treatment.
Blanks indicate that EPA has not derived TWFs for these chemicals. EPA does not assign toxicity values to
conventional pollutants or bulk parameters; therefore, these chemicals do not have TWFs.
D-2

Drinking Water Industry Report Appendix E
APPENDIX E
NATIONAL ESTIMATES: WATER TREATMENT PLANT COUNTS FOR POLLUTANT LOADINGS
ESTIMATES

Drinking Water Industry Report Appendix E
Table E-1. WTP Counts for Pollutant Loadings Excluding Chlorination Pollutants
Treatment Plant
Type
Solid/Water
Separation of
Residuals
Population Served
(Corresponds to
Discharge Flow Rate)
National Estimates
(Number of WTPs)
Direct
Indirect
Both
Lime Softening
Yes
10,000 to 50,000
46
41
23
50,000 to 100,000
55
8
7
100,000 to 500,000
33
19
4
More than 500,000
6
2
0
No
10,000 to 50,000
31
42
0
50,000 to 100,000
2
10
0
100,000 to 500,000
8
2
6
More than 500,000
2
0
0
Coagulation &
Filtration
Yes
10,000 to 50,000
257
181
40
50,000 to 100,000
63
34
28
100,000 to 500,000
48
46
14
More than 500,000
4
4
4
No
10,000 to 50,000
36
203
0
50,000 to 100,000
4
22
4
100,000 to 500,000
8
4
0
More than 500,000
0
0
0
Filtration only
Yes
10,000 to 50,000
22
31
8
50,000 to 100,000
0
0
0
100,000 to 500,000
2
0
0
More than 500,000
0
2
0
No
10,000 to 50,000
0
28
0
50,000 to 100,000
0
2
0
100,000 to 500,000
0
0
0
More than 500,000
0
0
0
Desalting
Membrane
Yes
10,000 to 50,000
2
8
8
50,000 to 100,000
0
0
0
100,000 to 500,000
0
0
0
More than 500,000
0
0
0
No
10,000 to 50,000
4
4
8
50,000 to 100,000
0
2
0
100,000 to 500,000
0
2
2
More than 500,000
0
0
0
Ion Exchange &
Adsorption
Yes
10,000 to 50,000
19
0
0
50,000 to 100,000
0
0
0
100,000 to 500,000
0
6
0
More than 500,000
0
0
0
No
10,000 to 50,000
0
65
0
50,000 to 100,000
0
2
0
100,000 to 500,000
0
0
0
More than 500,000
0
0
0
E-1

Drinking Water Industry Report Appendix E
Table E-1. WTP Counts for Pollutant Loadings Excluding Chlorination Pollutants
Treatment Plant
Type
Solid/Water
Separation of
Residuals
Population Served
(Corresponds to
Discharge Flow Rate)
National Estimates
(Number of WTPs)
Direct
Indirect
Both
None
Yes
10,000 to 50,000
0
19
0
50,000 to 100,000
0
0
0
100,000 to 500,000
2
0
0
More than 500,000
0
0
0
No
10,000 to 50,000
19
0
0
50,000 to 100,000
0
0
0
100,000 to 500,000
0
0
0
More than 500,000
0
0
0
E-2

Drinking Water Industry Report Appendix E
Table E-2. WTP Counts for Pollutant Loadings Chlorination Pollutants
Treatment
Plant Type
Chlorination
Dechlorination of
Residuals
(in 2f and/or 2h)
Population Served
(Corresponds to
Discharge Flow Rate)
National Estimates
(Number of WTPs)
Direct
Indirect
Both
Lime
Softening
Yes
Yes
10,000 to 50,000
12
0
8
50,000 to 100,000
8
0
5
100,000 to 500,000
2
2
2
More than 500,000
2
0
0
No
10,000 to 50,000
66
83
15
50,000 to 100,000
42
17
2
100,000 to 500,000
37
15
8
More than 500,000
5
2
0
No
a
NA
NA
16
Coagulation
& Filtration
Yes
Yes
10,000 to 50,000
39
2
8
50,000 to 100,000
14
0
12
100,000 to 500,000
25
0
4
More than 500,000
4
0
2
No
10,000 to 50,000
221
356
28
50,000 to 100,000
48
49
16
100,000 to 500,000
27
48
6
More than 500,000
0
2
0
No
a
NA
NA
94
Filtration only
Yes
Yes
10,000 to 50,000
8
10
0
50,000 to 100,000
0
0
0
100,000 to 500,000
0
0
0
More than 500,000
0
0
0
No
10,000 to 50,000
15
47
8
50,000 to 100,000
0
0
0
100,000 to 500,000
0
0
0
More than 500,000
0
2
0
No
a
NA
NA
7
Desalting
Membrane
Yes
Yes
10,000 to 50,000
0
0
0
50,000 to 100,000
0
0
0
100,000 to 500,000
0
0
0
More than 500,000
0
0
0
No
10,000 to 50,000
0
8
6
50,000 to 100,000
0
0
0
100,000 to 500,000
0
0
0
More than 500,000
0
0
0
No
a
NA
NA
27
E-3

Drinking Water Industry Report Appendix E
Table E-2. WTP Counts for Pollutant Loadings Chlorination Pollutants
Treatment
Plant Type
Chlorination
Dechlorination of
Residuals
(in 2f and/or 2h)
Population Served
(Corresponds to
Discharge Flow Rate)
National Estimates
(Number of WTPs)
Direct
Indirect
Both
Ion Exchange
& Adsorption
Yes
Yes
10,000 to 50,000
19
0
0
500,00 to 100,000
0
0
0
100,000 to 500,000
0
0
0
More than 500,000
0
0
0
No
10,000 to 50,000
0
57
0
50,000 to 100,000
0
0
0
100,000 to 500,000
0
0
0
More than 500,000
0
0
0
No
a
NA
NA
16
None
Yes
Yes
10,000 to 50,000
0
0
0
50,000 to 100,000
0
0
0
100,000 to 500,000
0
0
0
More than 500,000
0
0
0
No
10,000 to 50,000
19
19
0
50,000 to 100,000
0
0
0
100,000 to 500,000
2
0
0
More than 500,000
0
0
0
No
a
NA
NA
0
a – For plants that do not add chlorine, EP A assumes that pollutant loadings of chemicals from chlorination, such as
disinfection by-products, are zero.
E-4
